By way of background, Dr. Feldman highlighted that approximately 20 to 30% of patients with advanced germ cell tumors require salvage chemotherapy, with options including conventional-dose chemotherapy (DCT, ifosfamide + cisplatin + a third drug) or high-dose chemotherapy (high-dose chemotherapy (HDCT), carboplatin + etoposide based). The optimal of these options is not clear.
The IT-94 trial compared these two approaches finding a non-significant trend in improved event-free survival with HDCT but no difference in overall survival. However, Dr. Feldman highlighted difficulties in interpreting IT-94 including that patients in the HDCT arm received only one high-dose cycle, patients with incomplete response were excluded, there was an unexpectedly high rate of toxic death in the HDCT arm, and, finally, more than one-quarter of patients assigned to HDCT didn’t receive it.
Since that time, a unified prognostic system to stratify patients prior to salvage chemotherapy has been proposed to parallel the International Germ Cell Cancer Collaborative Group (IGCCCG) system for patients prior to initial first-line therapy.

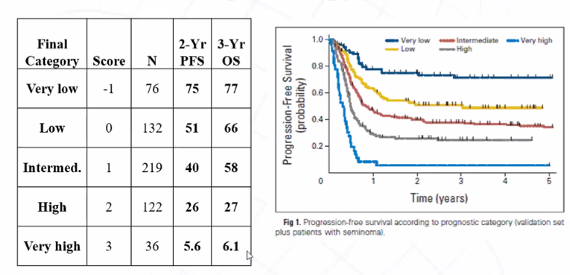
In observational data, use of HDCT is associated with significantly improved progression-free (absolute difference in 5-year PFS 22%; hazard ratio 0.44, p<0.001) and overall survival (absolute difference in 5-year OS 12%; hazard ratio 0.65, p<0.001). Further retrospective analyses from both Memorial Sloan Kettering Cancer Center and Indiana have supported these findings.
Thus, there are discordant results from large retrospective series and multiple small series and the one randomized controlled trial in this setting. Further, standard practice around the world differs substantially.
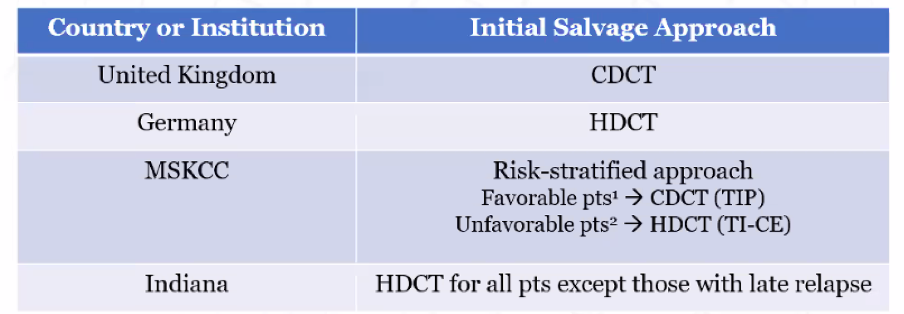
In this context, the TIGER trial was designed. Powered for overall survival, 420 patients (double IT-94) will be accrued and randomized to TIP conventional-dose chemotherapy or TI-CE high-dose chemotherapy.

The trial includes men aged 14 years and older with confirmed germ cell tumors of any primary site who have progressive disease following 1 line of cisplatin-based chemotherapy (at least 3 but no more than 6 cycles) with adequate organ function who are eligible for high-dose treatment per FACT (including HIV negative and Hepatitis B negative). Exclusion criteria include prior treatment with TIP or HDCT, concurrent cancer, and large or symptomatic brain metastases.
In arm A, patients will receive a TIP regime with paclitaxel (day 1), ifosfamide (days 2-5), cisplatin (days 2-5), and mesna support. In arm B, patients receive a TI-CE regime based on paclitaxel and ifosfamide in cycles 1-2 and carboplatin and etoposide in cycles 3-5.

As mentioned, the study’s primary endpoint was overall survival. The sample size of 420 patients is anticipated to be contributed from 168 patients in North America and 252 patients in Europe. The study was designed using a cure rate model assuming that 35% of patients will be cured and median survival time of 1.5 years among those treated with TIP. The authors assumed a hazard ratio of 0.71 to give 81% power for a one-sided type 1 error rate of 0.05.
Secondary endpoints include progression-free survival, validation of the International Prognostic Factor Study Group, toxicity including treatment-related mortality, pharmacogenomics associated with platinum response, whole-exome sequencing, and quality of life metrics.
Dr. Feldman emphasized that this study is important given that there is no standard of care in this young, curable population. Further, this trial will improve outcomes regardless of result.

Additionally, this study will provide a large biobank of tumors, external validation of the IPFSG model, and evaluation of quality of life metrics.
To date, 314 of a targeted 420 patients have been accrued with an average of 8 pre months, prior to COVID. Since COVID, approximately 5 patients per month have been accrued. Thus, completion of accrual has likely deferred to mid-2022.

Presented by: Darren Feldman, MD, Medical Oncologist, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York
Written by: Christopher J.D. Wallis, MD, PhD, FRCSC, Instructor in Urology, Vanderbilt University Medical Center, Nashville, Tennessee Contact: @WallisCJD on Twitter during the 1st Global Society of Rare Genitourinary Tumors Virtual Summit, December 11-12, 2020


