Establishing the context for his talk, Dr. Bagrodia emphasized that testicular cancer is highly curable across all disease states. However, necessary interventions have significant toxicity. Thus, moving forward, it is paramount to minimize toxicity while maintaining excellent oncologic outcomes.
Dr. Bagrodia then took a stage-specific look at interventions and associated toxicity. Beginning with clinical stage I (CSI) disease treated by orchiectomy, he emphasized the importance of the testicle in androgen production. Following orchiectomy between 10-20% of patients have laboratory evidence of hypogonadism which may be associated with a range of adverse psychosocial, physical, and reproductive effects.
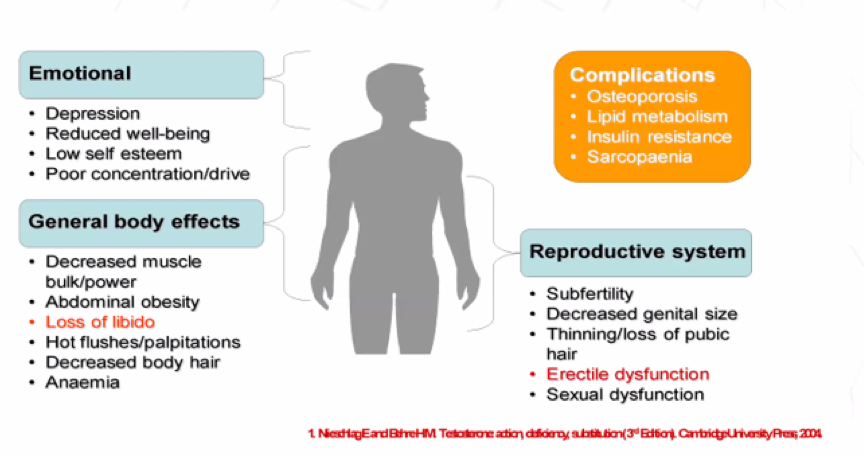
Further, subfertility and infertility are common among men with testicular cancer prior to orchiectomy, though post-orchiectomy paternity is high (90%). Dr. Bagrodia emphasized the important psychosocial effects of orchiectomy including feelings of loss, shame or uneasiness, clinically significant anxiety, clinically significant depression, and fear of recurrence.
Thus, he recommended that clinicians should have an early discussion of the effects of orchiectomy and germ cell tumor diagnosis, including cryopreservation and testicular prosthesis. Moving forward, patients should have an ongoing assessment and appropriate medical care to identify and treat issues as they arise.
In the context of stage I/II disease, he highlighted complications of primary retroperitoneal lymph node dissection (RPLND), including intra-operative, post-operative, and late effects. Potentially the most meaningful of these, in his view, was rates of anejaculation ranging from 1 to 50%.
In the context of adjuvant chemotherapy, he highlighted dose-related, non-dose-related, and uncertain toxicities of treatment.

For patients receiving adjuvant carboplatin for CSI seminoma, there is a lack of long-term toxicity data, though there is an apparent increase in CV risk and gonadal dysfunction.
Radiotherapy for stage I/II seminoma is associated with both short-term adverse events and long-term risks of secondary malignancy and cardiovascular complications.
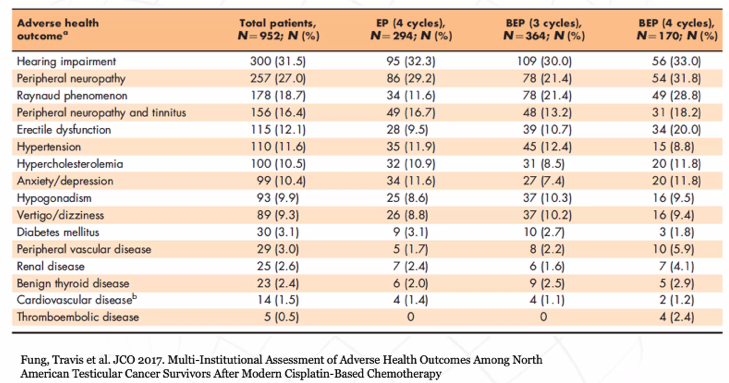
Beyond these physician adjudicated complications, Dr. Bagrodia emphasized how common self-reported adverse health outcomes are. Among patients receiving chemotherapy, hearing impairment is present in nearly one-third and many other significant complications are also common.
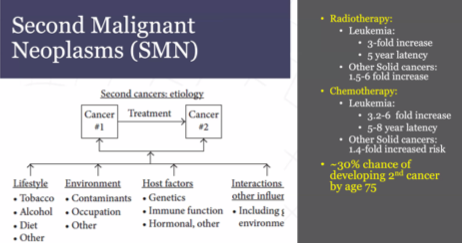
Assessing specific treatment-related morbidity, Dr. Bagrodia first highlighted the risk of secondary malignancies. These may occur following either radiotherapy (in-field solid organ cancers and leukemias) and chemotherapy (again, both leukemias and solid organ cancers). Among patients with germ cell tumors, the risk of a second cancer is 30% by age 75 with contributions from lifestyle, environment, host genetic and hormonal factors, and treatment contributing.
Dr. Bagrodia then discussed the risk of cardiovascular disease. Again, this is elevated for patients following both chemotherapy (1.5 – 7x relative risk and absolute rate of 7-18% among patients with germ cell tumors) and following radiotherapy (1.4 – 2.4x relative risk). Neurotoxicity occurs in 20-40% of patients following chemotherapy due to degeneration of the dorsal root ganglion with a generally unknown natural history. Similarly, ototoxicity affects approximately 1 in 5 who report tinnitus or high-frequency hearing loss; this is due to damage to the outer hair cells of the cochlea from chemotherapy. Nephrotoxicity is another potential consequence of chemotherapy and may manifest with either acute or chronic renal dysfunction and dose-dependent decreases in GFR due to proximal and distal renal tubular dysfunction. While pulmonary toxicity is feared, rates are relatively low with modern chemotherapy administration though somewhat higher among smokers, those with prior pulmonary surgery, pulmonary emboli, or poor-risk disease.
Taken together, the consequences of treatment of testicular cancer means that these patients have a 6% risk of excess morbidity compared to the general population despite very high cancer cure rates.
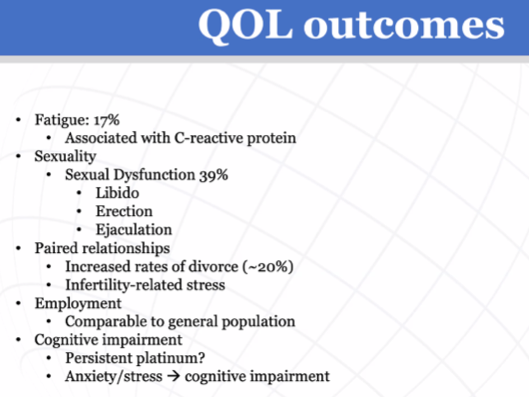
Dr. Bagrodia then discussed quality of life outcomes, based on data from SWENOTECA, including issues related to fatigue, sexuality, paired relationships (including increased rates of divorce), and long-term cognitive impairment.
In conclusion, Dr. Bagrodia emphasized that there are many significant late effects of testicular cancer treatment, including cardiovascular disease, secondary cancers, neurotoxicity, nephrotoxicity, pulmonary toxicity, hypogonadism, impaired fertility, psychosocial disorders, cognitive impairments, and quality of life effects. Moving forward, he suggested that there is an ongoing need for research into late effects of testicular cancer treatment including understanding the mechanisms of toxicity and susceptibility to late effects (including genetic susceptibility studies using GWAS). In terms of practical implementation, he emphasized that the clinical standard of care is screening for adverse effects, which may be facilitated by standardized intake questions with quality of life assessment.
Presented by: Aditya Bagrodia, MD, Assistant Professor, Department of Urology, UT Southwestern Medical Center, Dallas, Texas
Written by: Christopher J.D. Wallis, MD, PhD, FRCSC, Instructor in Urology, Vanderbilt University Medical Center, Nashville, Tennessee Contact: @WallisCJD on Twitter during the 1st Global Society of Rare Genitourinary Tumors Virtual Summit, December 11-12, 2020


