(UroToday.com) PURE-01 was an open-label, single-arm, phase 2 trial where three courses of 200mg pembrolizumab were administered in T2-4aN0M0 bladder cancer patients preceding radical cystectomy (RC).1 A proportion of these patients had residual invasive disease (ypT2-4) at radical cystectomy. Investigators from Fondazione IRCCS Istituto Nazionale dei Tumori explored the biological characteristics of pembrolizumab-resistant tumors in the context of this trial.
They performed gene expression profiling on 26 RC samples from patients with ypT2-4 disease post-pembrolizumab, of which 22 had matched pre-pembrolizumab transurethral tumor resection samples. Matched cases were analyzed by differential gene expression, hallmark signatures, and molecular subtyping. Unsupervised consensus clustering was used to compare the 26 post-pembrolizumab samples with independent post-standard neoadjuvant chemotherapy samples, and other samples collected from the former tumor bed of neoadjuvant chemotherapy-treated patients (scar tissue), all derived from RC specimens.
Initial molecular subtyping of the pre- and post-pembrolizumab samples revealed significant subtype switching. Unsupervised consensus clustering analysis revealed a “scar-like” molecular subtype at radical cystectomy, likely induced by transurethral tumor resection that generated a wound healing response. When all samples were characterized genomically, those who underwent cystectomy with or without cisplatin-based chemotherapy exhibited such scar-like features. However, the majority of cases from the PURE-01 cohort expressed luminal markers, suggesting that pembrolizumab may select for, or induce, a luminal phenotype in residual tumor tissue. With systemic therapy, the scar-like subtype demonstrated a better prognosis (Figure 1).
Figure 1: Scar-like subtype has improved prognosis.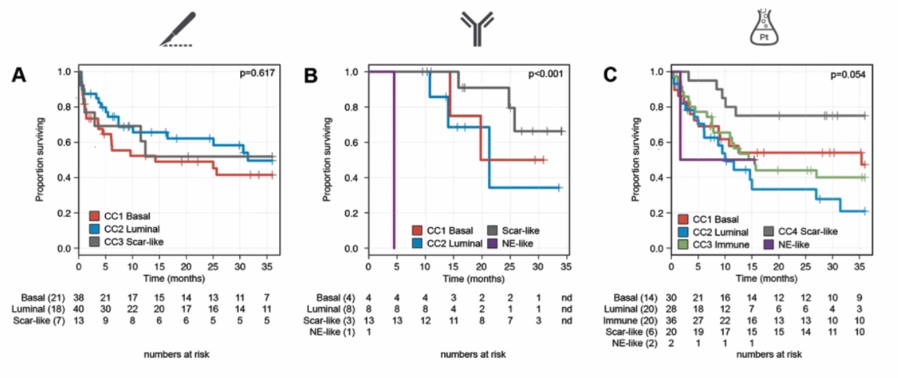
With the combined characterization, the authors identified several potential therapeutic targets in the tumor tissue in RC specimens (Figure 2). Specifically for patients treated with neoadjuvant pembrolizumab, adjuvant treatment decisions may focus on targeted therapies for the luminal subtype.
Figure 2: Characterization of residual tumors on radical cystectomy specimens.
Presented by: Joep J. de Jong, MD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Written by: Anirban P. Mitra, MD, Ph.D., Urologic Oncology Fellow, The University of Texas MD Anderson Cancer Center, Houston, TX, USA, Twitter: @APMitra, with Ashish M. Kamat, MD, MBBS, President of IBCN and IBCG, Endowed Professor, The University of Texas MD Anderson Cancer Center, Houston, TX, USA, Twitter:@UroDocAsh, at the International Bladder Cancer Network (IBCN) Annual Meeting, #IBCN2020, October 17, 2020.
References:
1. Necchi A, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol. 2018;36:3353-3360.


