(UroToday.com) The 2024 IBCN annual meeting included a bladder cancer session, featuring a presentation by Dr. Andrea Necchi discussing results from the multicenter OPTIMUS umbrella study assessing retifanlimab monotherapy as neoadjuvant therapy for patients with cisplatin-ineligible muscle-invasive bladder cancer. Muscle invasive urothelial carcinoma of the bladder has a high recurrence rate and modest improvements are seen with neoadjuvant chemotherapy over radical cystectomy, necessitating more effective neoadjuvant therapy. Intratumoral CD8+ T-cell count has been shown to positively correlate with disease-free survival and overall survival in patients with muscle invasive urothelial carcinoma of the bladder. Pathological downstaging is a potential surrogate marker of efficacy and increased survival for muscle invasive bladder cancer neoadjuvant therapies. The anti-PD1 monoclonal antibody retifanlimab is approved in the US and European Union for the treatment of Merkel cell carcinoma and has demonstrated preliminary efficacy in cisplatin-ineligible locally advanced/metastatic urothelial cancer in the phase 2 POD1UM-203 study. Retifanlimab monotherapy achieved an overall response rate of 38% and disease control rate of 55% in 29 patients with locally advanced/metastatic urothelial carcinoma and PD-L1 CPS >= 10. At IBCN 2024, Dr. Necchi and colleagues reported results from patients receiving neoadjuvant retifanlimab monotherapy for muscle invasive bladder cancer in the OPTIMUS study.
This was an open-label, randomized, phase 2, window-of-opportunity, platform study in adults with stage T2-3b, N0M0 muscle invasive bladder cancer prior to radical cystectomy who were ineligible or refused cisplatin-based neoadjuvant therapies, and had ECOG performance status ≤1. Patients were stratified by pretreatment biopsy PD-L1 combined positive score (CPS ≥10 or <10) and randomized to receive 5 different treatments, including retifanlimab (500 mg q4w) as monotherapy or in combination with other immunotherapies, for 4-10 weeks before cystectomy. The OPTIMUS study design was as follows:

Patients in group B received retifanlimab 500 mg every 4 weeks as monotherapy for a maximum of 3 cycles. The primary endpoint was change in tumor CD8+ lymphocyte count at cystectomy versus pretreatment. Secondary endpoints included frequency and severity of treatment-emergent adverse events, pathologic complete response, and major pathologic response, defined as ypT0N0 and residual ypT0/1/a/isN0M0, respectively. Tumor biopsies were collected prior to enrollment at the last tumor resection, and at the time of cystectomy, for tumor infiltrating CD8+ T-effector cell assessment. Pathological response to treatment was assessed based on histological evaluation of the TURBT and cystectomy samples by local institutional analysis.
As of January 29, 2024, 20 patients had received retifanlimab monotherapy (staging: cT2, 85%; cT3, 10%; unknown, 5%). The median age was 73 (range: 62-82) years, 90% were male, 90% were ECOG performance status 0, and 55% were PD-L1 CPS ≥10:
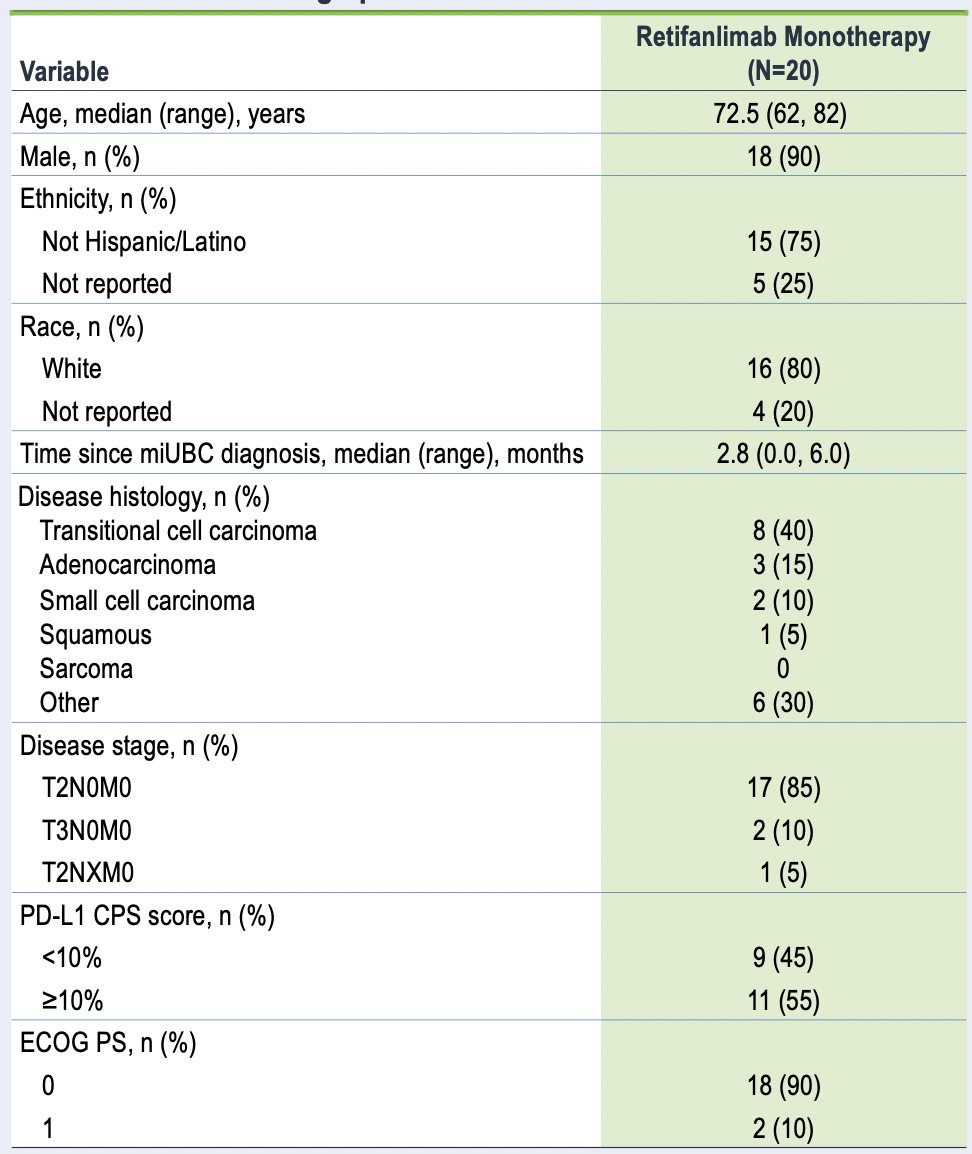
Patients received a median of 3 (range: 1-3) cycles of retifanlimab treatment, with a median duration of exposure of 57 (range: 1-60) days, and 1 patient with dose delay. Out of 20 treated patients, 17 completed treatment (of which 15 received 3 cycles of retifanlimab infusions) before cystectomy, 3 discontinued treatment due to safety concerns, and all 20 patients underwent radical cystectomy. Change in tumor CD8+ lymphocyte count at cystectomy was evaluable in 6 patients, with a mean fold change from baseline was 0.79 (80% CI 0.23-1.35; p = 0.09), and the minimum and maximum fold changes were -0.67 and 1.92, respectively:

At time of cystectomy assessment, 10 patients had a major pathologic response (50%, 80% CI 34-66) and 8 had pathologic complete response (40%, 80% CI 25-57). Patients with pathologic complete response were mutually exclusive for primary endpoint assessment. Treatment-emergent adverse events were noted in 18 patients (90%), the most common (occurring in >=20% of patients) were anemia and pyrexia (25% each) and hypertension (20%). Treatment emergent adverse events that led to discontinuation in 2 patients (1 syncope, 1 myositis) and delayed cystectomy in 1 patient (diarrhea). Two patients had immune-related treatment-emergent adverse events (1 rash, 1 myalgia, myositis [grade 3] with elevated aspartate aminotransferase, blood creatinine phosphokinase, lactate dehydrogenase). No fatal treatment-emergent adverse events were reported:

Dr. Necchi concluded her presentation by discussing results from the multicenter OPTIMUS umbrella study assessing retifanlimab monotherapy as neoadjuvant therapy for patients with cisplatin-ineligible muscle-invasive bladder cancer with the following take-home points:
- Retifanlimab 500 mg every four week monotherapy was generally well tolerated and demonstrated promising efficacy in patients with stage T2-3bN0M0 muscle-invasive urothelial carcinoma of the bladder prior to radical cystectomy who were ineligible or refused cisplatin-based neoadjuvant therapies
- The pathologic complete response rate was 40% and the major pathologic response rate was 50%, limiting the evaluation of the primary endpoint
- The safety and tolerability profile of retifanlimab monotherapy was consistent with previous slides of retifanlimab and characteristic of the PD-(L)-1 inhibitor class
- Further investigation is warranted of retifanlimab as neoadjuvant therapy for cisplatin-ineligible patients with muscle-invasive urothelial carcinoma of the bladder who are candidates for radical cystectomy
Presented by: Andrea Necchi, MD, San Raffaele Hospital, Milan, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024.


