(UroToday.com) The 2024 IBCN annual meeting included a session on advancements in circulating biomarkers, featuring a presentation by Dr. Lourdes Mengual discussing dynamic monitoring of circulating tumor DNA (ctDNA) to predict prognosis in muscle-invasive bladder cancer patients after radical cystectomy. ctDNA has recently emerged as a real-time prognostic and predictive biomarker for monitoring cancer patients, particularly as an early indicator of tumor recurrence and treatment efficacy:
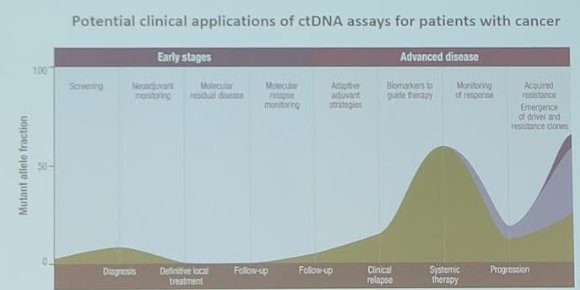
In this study, Dr. Mengual and colleagues assessed the prognostic value of ctDNA status for monitoring disease progression and therapeutic response in muscle-invasive bladder cancer patients at various follow-up time points after radical cystectomy. Additionally, they investigated whether tumor-agnostic ctDNA testing could be a feasible strategy for monitoring these patients.
Two cohorts of muscle-invasive bladder cancer patients who underwent radical cystectomy were evaluated:
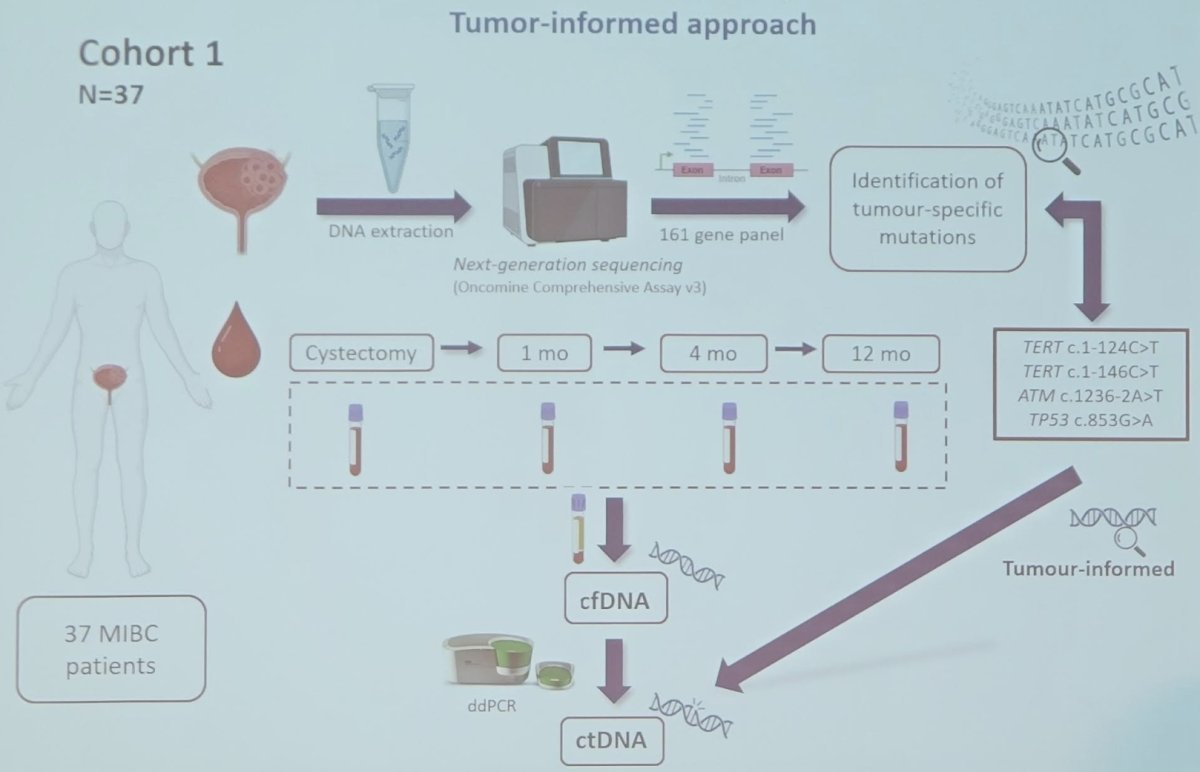
- Cohort 1 (37 patients): underwent tumor-informed testing
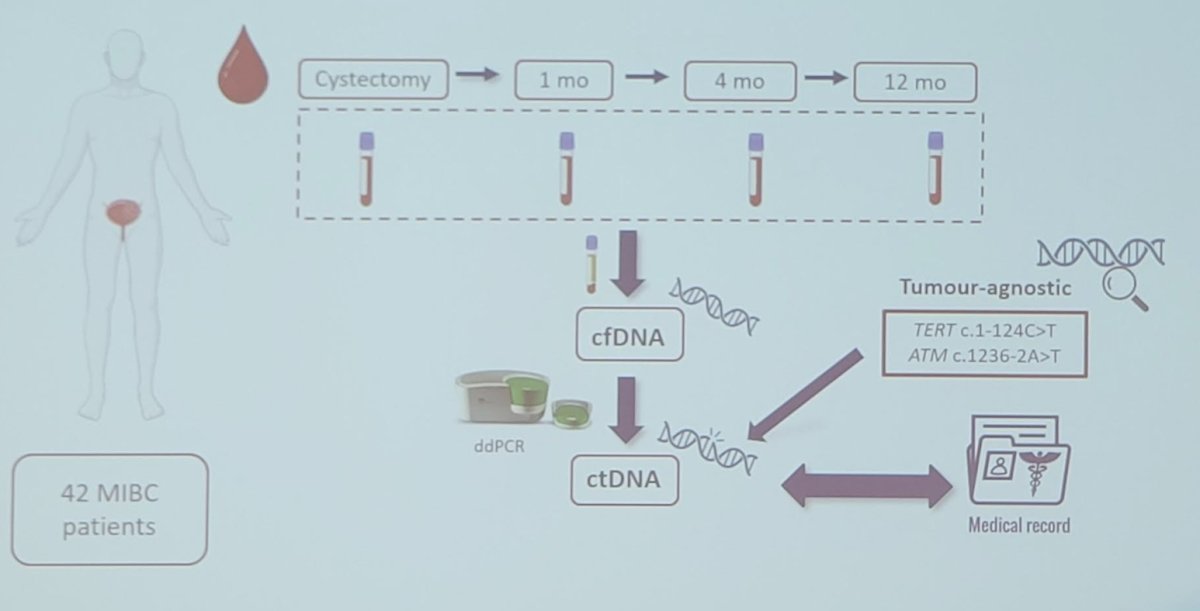
- Cohort 2 (42 patients): was analyzed using a tumor-agnostic approach
Blood samples were collected from patients at cystectomy and at 1, 4, 12, and 24 months post-surgery. Cell-free DNA (cfDNA) was extracted from plasma samples and ctDNA status was determined in each patient and at each follow-up time point using droplet digital PCR. In cohort 1, four mutations previously identified in primary tumor tissue were determined while in cohort 2, two out of the four specific mutations analyzed in cohort 1 were assessed using a tumor-agnostic approach.
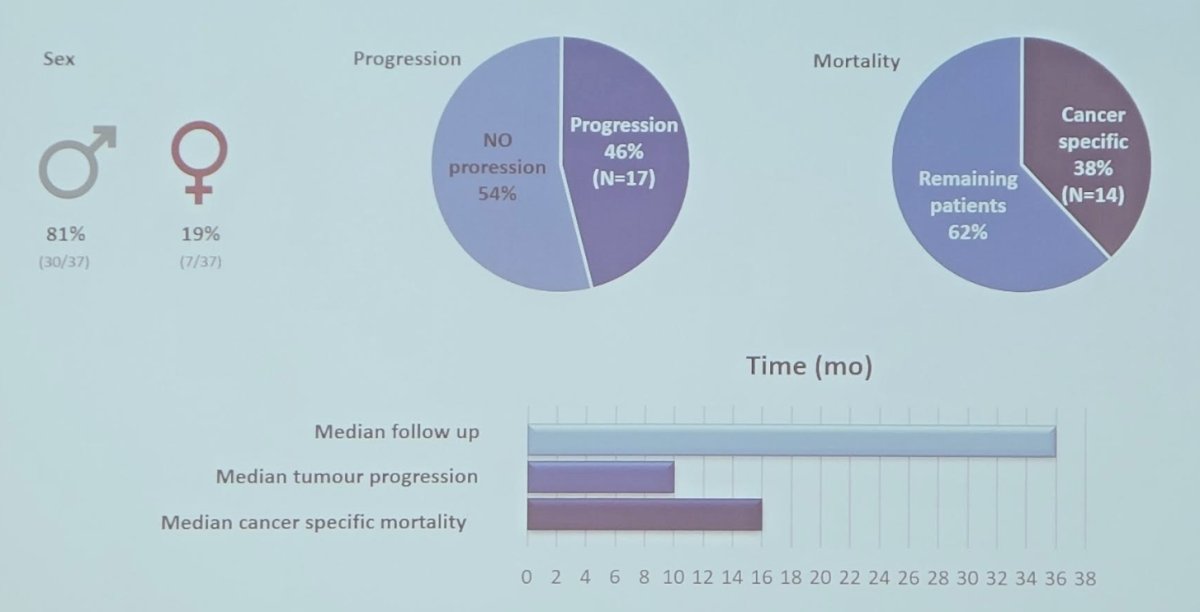
For Cohort 1, over a median follow-up of 36 months, 46% of patients progressed, with a median time to progression of 10 months:
In cohort 1, there was 65% mutation concordance between tissue samples and blood samples (ctDNA). Additionally, higher pT stage at cystectomy was associated with more ctDNA positive samples. ctDNA status four months after radical cystectomy was identified as an independent prognostic biomarker of cancer-specific survival (HR 4.199; p = 0.038):
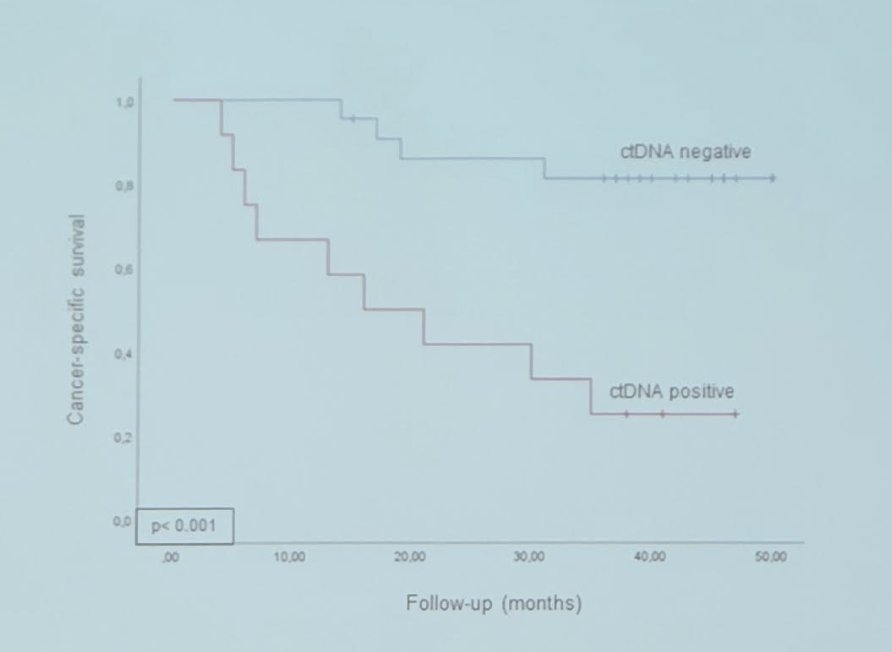
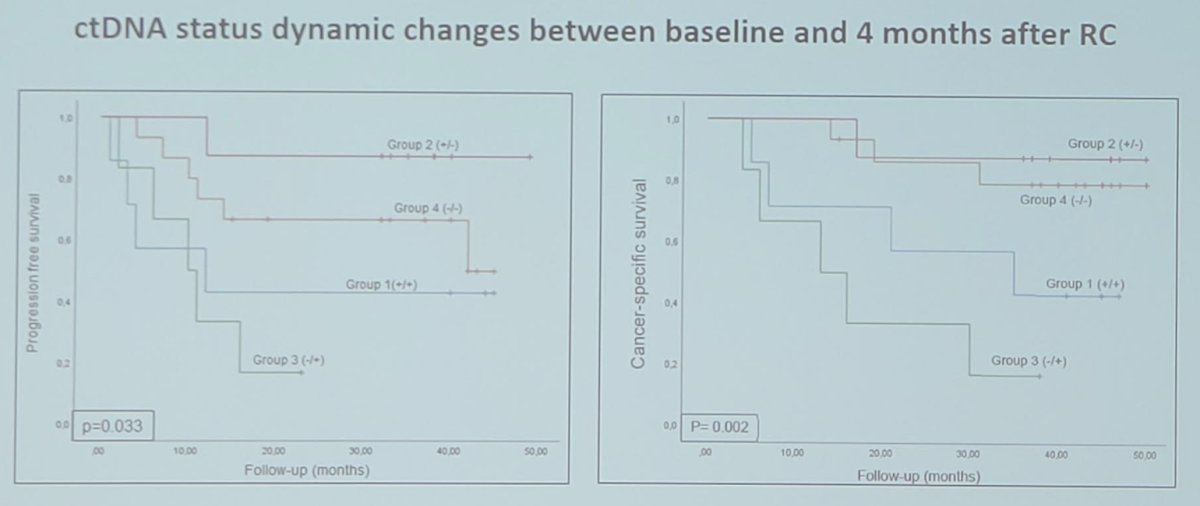
Furthermore, ctDNA clearance four months after radical cystectomy was significantly associated with clinical outcomes:
For Cohort 2, over a median follow-up of 21 months, 24% of patients progressed, with a median progression time of six months:
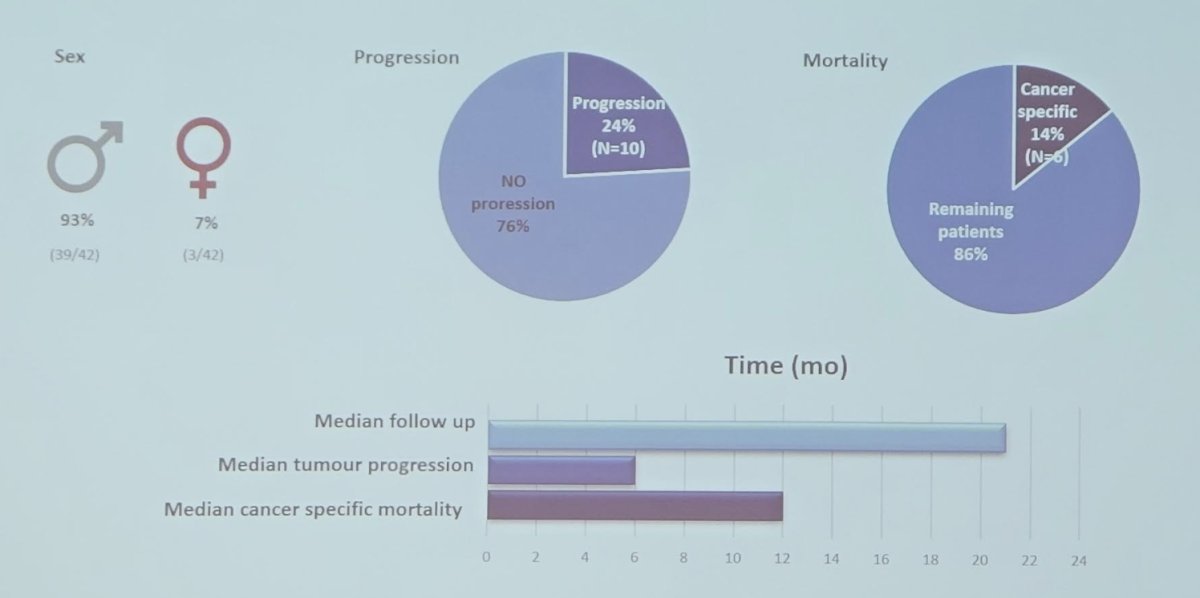
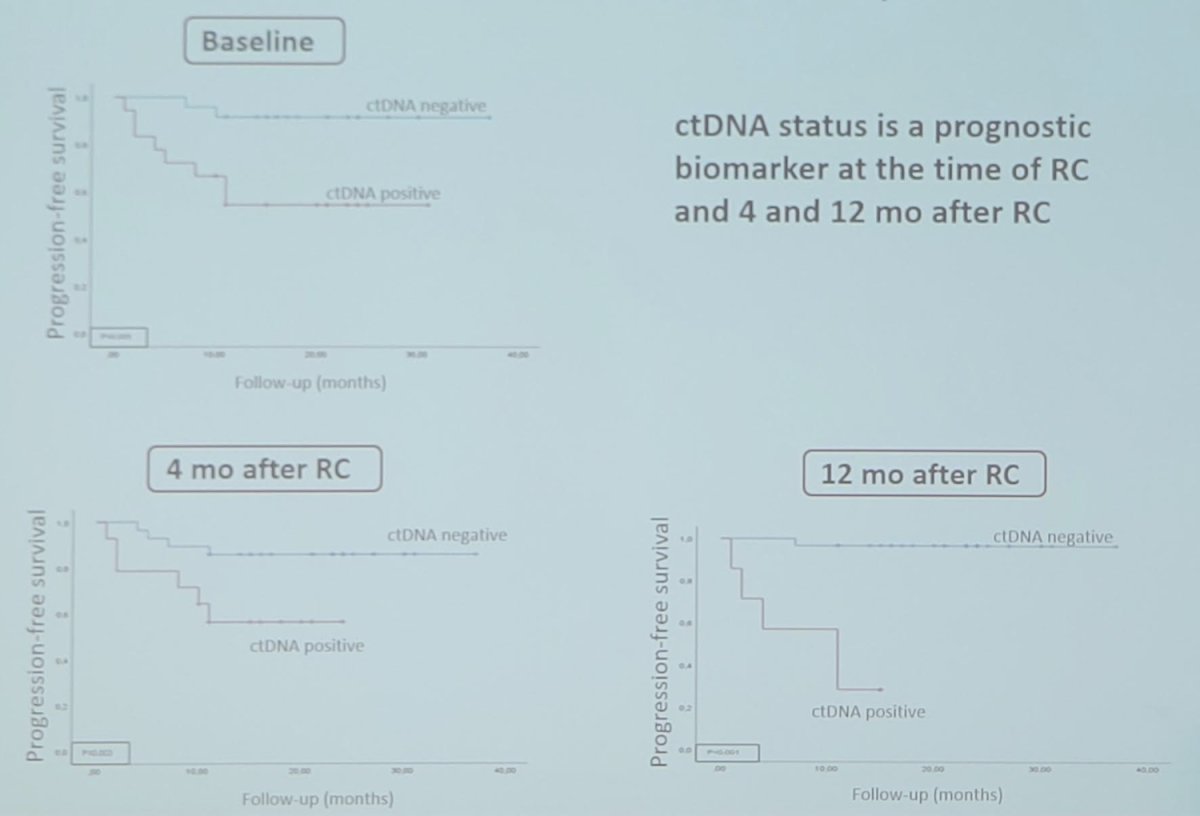
ctDNA status was identified as a prognostic biomarker of tumor progression before radical cystectomy (HR 6.774, p < 0.05), and at 4 (HR 3.673, p < 0.05), and 12 months post-surgery (HR 30.865, p < 0.05):
Additionally, dynamic changes in ctDNA status between baseline and four months later were significantly associated with patient outcomes (p = 0.045).
Dr. Mengual concluded her presentation by discussing dynamic monitoring of ctDNA to predict prognosis in muscle-invasive bladder cancer patients after radical cystectomy with the following take-home points:
- ctDNA status is a useful biomarker of tumor progression and cancer specific survival in muscle invasive bladder cancer patients after radical cystectomy
- ctDNA status is able to detect tumor progression earlier than imaging techniques
- Changes in ctDNA status has prognostic implications in muscle invasive bladder cancer patients after radical cystectomy
- ctDNA analysis using a tumor agnostic approach can be a powerful tool for monitoring muscle invasive bladder cancer patients after radical cystectomy
- The implementation of cfDNA analysis in the clinical setting could have an impact on disease management and patient clinical outcomes
Presented by: Lourdes Mengual, PhD, Universitat de Barcelona, Barcelona, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024.