(UroToday.com) The 2024 IBCN annual meeting included a session on advancements in circulating biomarkers, featuring a presentation by Dr. Kent Mouw discussing the correlation of ctDNA dynamics with clinical response in muscle-invasive bladder cancer patients undergoing trimodality therapy. ctDNA is defined as small fragments of DNA released from dying tumor cells, has a short half-life, and can be detected by NGS and other techniques. Additionally, ctDNA can be used for tumor detection, surveillance, and response, as well as with increasing indications across multiple tumor types.
The Signatera assay is a commercially available, tumor-informed ctDNA assay approved for use in several cancer settings in the United States, and for bladder cancer is approved by Medicare in the peri-operative and surveillance settings: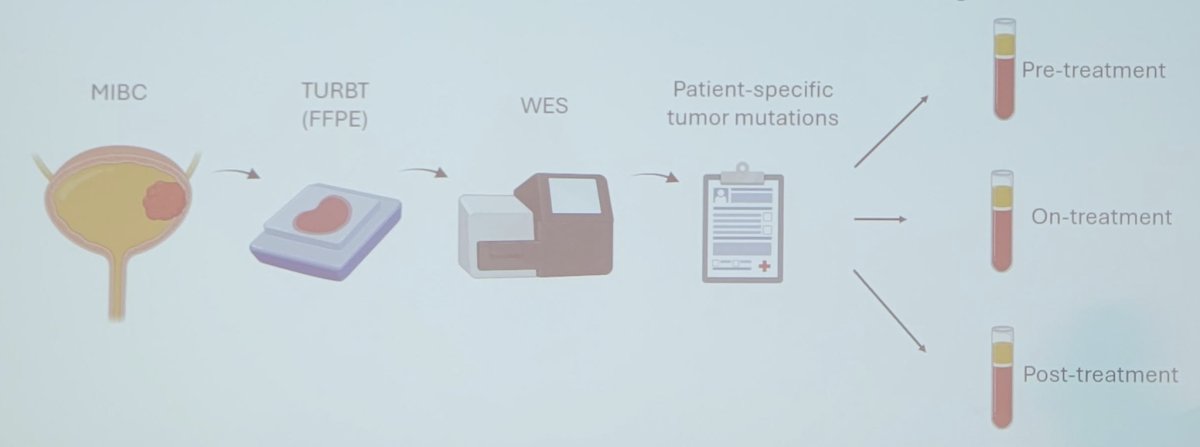
Trimodality therapy is a curative-intent treatment option for patients with muscle-invasive bladder cancer, supported by long-term safety and efficacy data, as well as guidelines. Recently, plasma ctDNA has been associated with treatment response and clinical outcomes following cystectomy for bladder cancer, but the association of plasma ctDNA with treatment response and clinical outcomes in patients with muscle-invasive bladder cancer treated with trimodality therapy is poorly understood. Dr. Mouw and colleagues hypothesized that ctDNA dynamics are correlated with clinical outcomes in muscle-invasive bladder cancer patients treated with trimodality therapy.
Select patients with muscle-invasive bladder cancer who received trimodality therapy at Dana-Farber/Brigham and Women’s Cancer Center between May 2023 and September 2024 who underwent pre-trimodality therapy ctDNA evaluation with the commercially available Signatera assay were included in this analysis. Individual chart review was performed to collect demographic and clinical data including disease stage, histopathology, imaging, and treatment details.
Pre-treatment ctDNA results were available for 30 patients. The baseline characteristics for this cohort are as follows: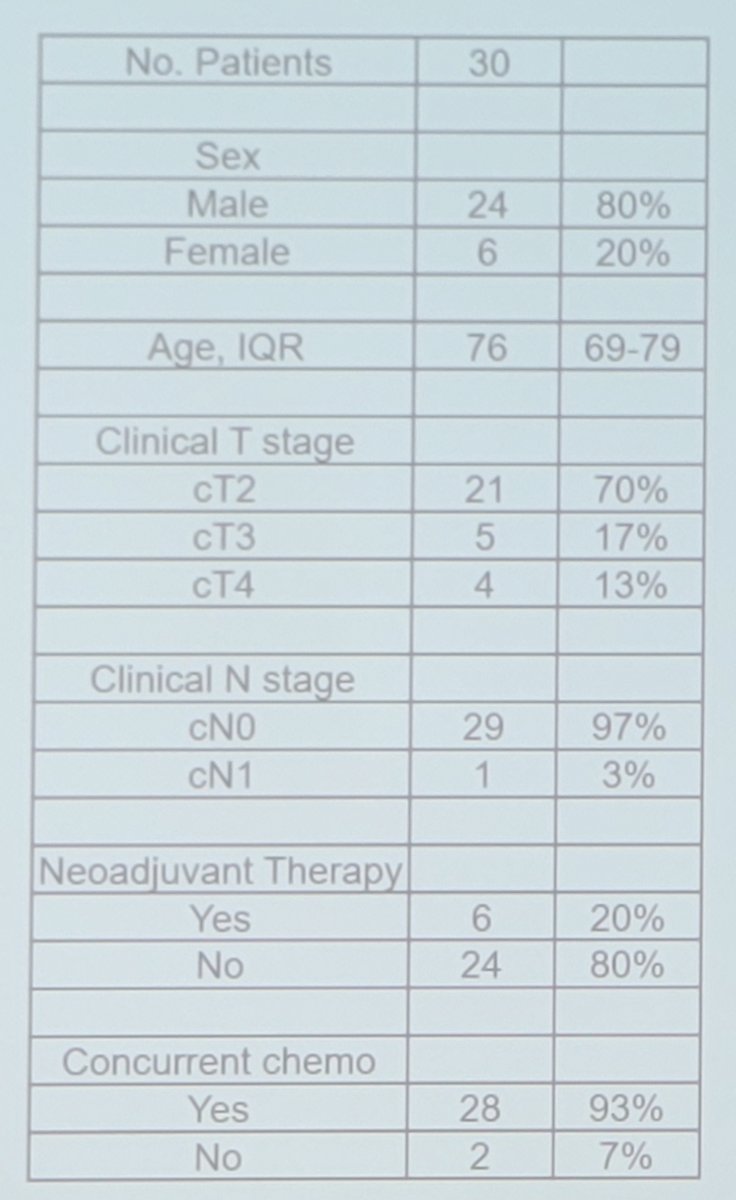
There were 29 of 30 patients that had at least one ctDNA test performed prior to starting bladder radiotherapy, with 11 of 29 (38%) having detectable ctDNA prior to trimodality therapy initiation. This included 9/20 positive with cT2N0 disease, and 5/9 positive with cT3/4 or cN1 disease. Moreover, among these 29 patients, 6 (21%) patients with a pre-radiotherapy ctDNA test received neoadjuvant therapy prior to bladder radiotherapy: 3/6 were ctDNA positive and 3/6 were ctDNA negative pre-neoadjuvant therapy, with all 6 patients being ctDNA negative after neoadjuvant therapy. There were 22 patients that had at least one pre-radiotherapy ctDNA test and at least one post-radiotherapy test, of which 5 of the 9 patients converted to having undetectable ctDNA following trimodality therapy and are free of disease, as assessed by routine post-trimodality therapy surveillance. Four patients had persistent detectable ctDNA following trimodality therapy: two with radiographic evidence of new metastatic disease on first post-trimodality therapy imaging, one without evidence of recurrence on first post-trimodality therapy assessment (imaging and cystoscopy), and one awaiting first post-trimodality therapy assessment: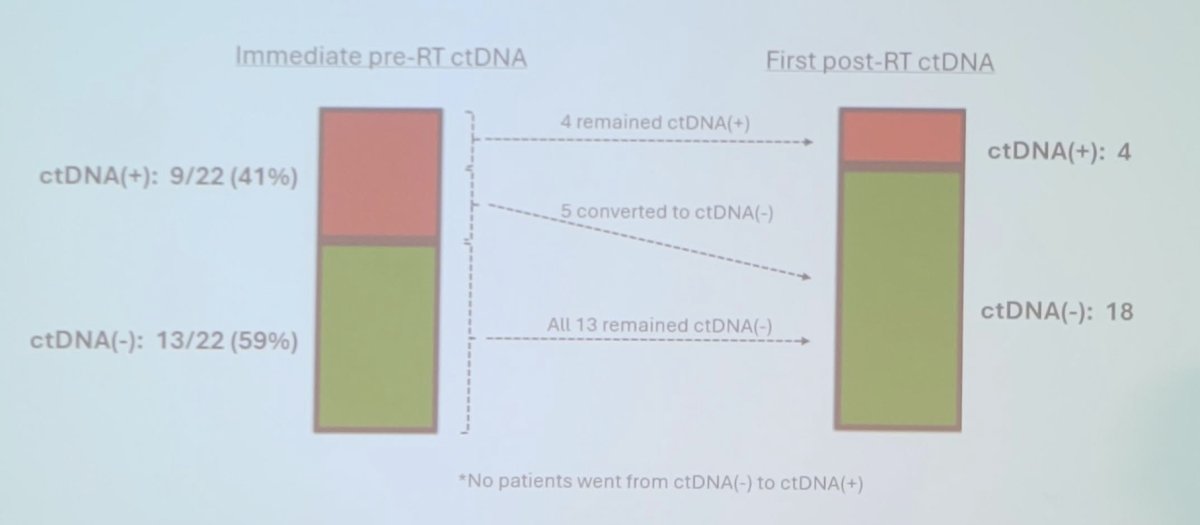
Among 18 patients with a first post-radiotherapy ctDNA negative, 17/18 (94%) have remained ctDNA negative, over a median follow-up of 7.5 months. None of the patients with persistent ctDNA negative have confirmed recurrent muscle-invasive bladder cancer or distant metastasis; 1 patient with cT4 tumor received avelumab and the rest have been on surveillance. None of the 5 patients who converted from ctDNA positive before radiotherapy to ctDNA negative after radiotherapy have experienced a recurrence.
Dr. Mouw noted several limitations of the current study:
- Retrospective in nature
- Limited size (but increasing)
- Limited follow-up to date
- Heterogeneous clinical characteristics and treatments (but all patients received definitive dose bladder radiotherapy)
- There were too few events to understand whether ctDNA conversion can impact disease course and clinical outcomes
Dr. Mouw concluded his presentation discussing the correlation of ctDNA dynamics with clinical response in muscle-invasive bladder cancer patients undergoing trimodality therapy with the following take-home points:
- This is one of the first and largest descriptions of plasma ctDNA dynamics following curative intent bladder radiotherapy in patients with muscle-invasive bladder cancer
- Approximately 40% of patients were ctDNA positive prior to trimodality therapy in this cohort
- Approximately 50% of patients with ctDNA positive prior to trimodality therapy converted to ctDNA negative after trimodality therapy
- Patients with persistent ctDNA positive after trimodality therapy suffered early metastatic recurrence
- Patients with ctDNA negative after trimodality therapy are doing well (caveat – limited follow-up to date)
Future directions include:
- Continuing to expand retrospective cohort numbers, and extend follow-up time
- Analyze samples from prospectively collected cohorts and trials
- Integrate plasma ctDNA data with other clinical and genomic features including urine ctDNA
Presented by: Kent Mouw, MD, PhD, Radiation Oncologist, Dana Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024


