(UroToday.com) The 2024 IBCN annual meeting included a session on emerging technologies in bladder cancer, featuring a presentation by Dr. Andrea Necchi discussing the value of mpMRI in assisting decision-making in muscle-invasive bladder cancer.
Dr. Necchi notes that in the application of novel imaging modalities in muscle-invasive bladder cancer, it is a stepwise approach: from improving disease staging to improving response assessment, to identifying the outlier responders, and to anticipating tumor relapse post-surgery: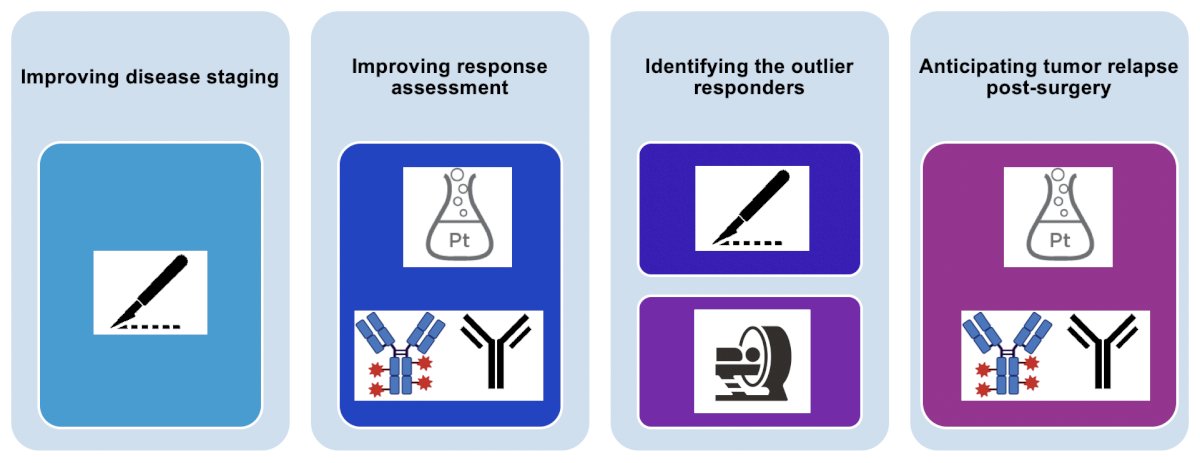
The following timeline highlights the steps towards a noninvasive diagnosis of muscle-invasive bladder cancer, starting with MRI in 2000 having an overall accuracy of 56-62% but over-staging in 32-38% of patients. Fast forward to 2018, which was the introduction of the VI-RADS scoring system, and now to 2023/2024 with the modified VI-RADS system in the immune checkpoint inhibitor setting with validation of nacVI-RADS post-immune checkpoint inhibitor therapy: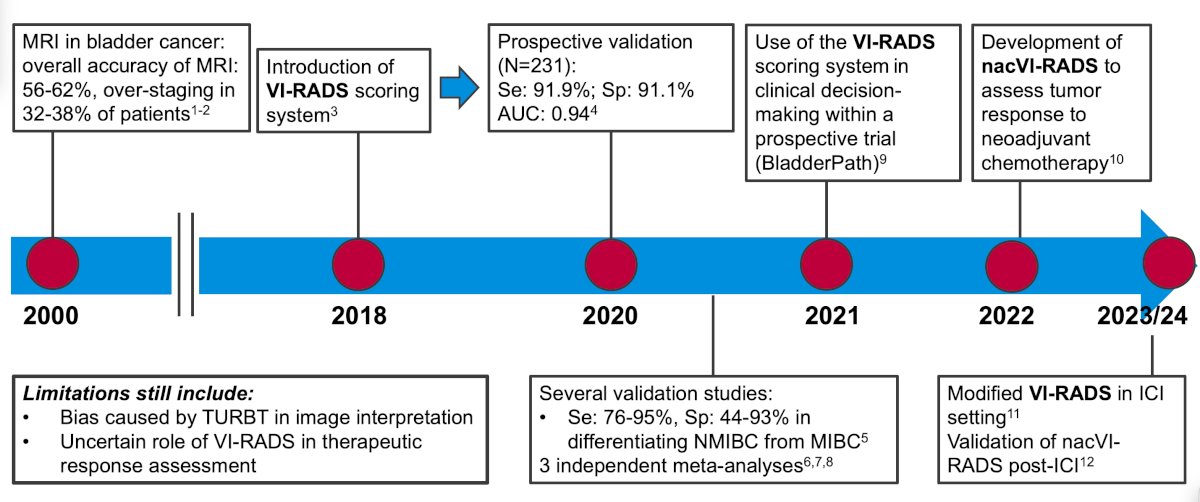
Multi-parametric MRI is a noninvasive way of diagnosing and assessing tumor response, which has been assessed in the PURE-01 trial (n = 164) [1]. Dr. Necchi highlighted that the PURE-01 study had an internal AUC of radiologic complete response for T0 of 0.76 (95% CI 0.68-0.83) and an external AUC of 0.74 (95% CI 0.66-0.82):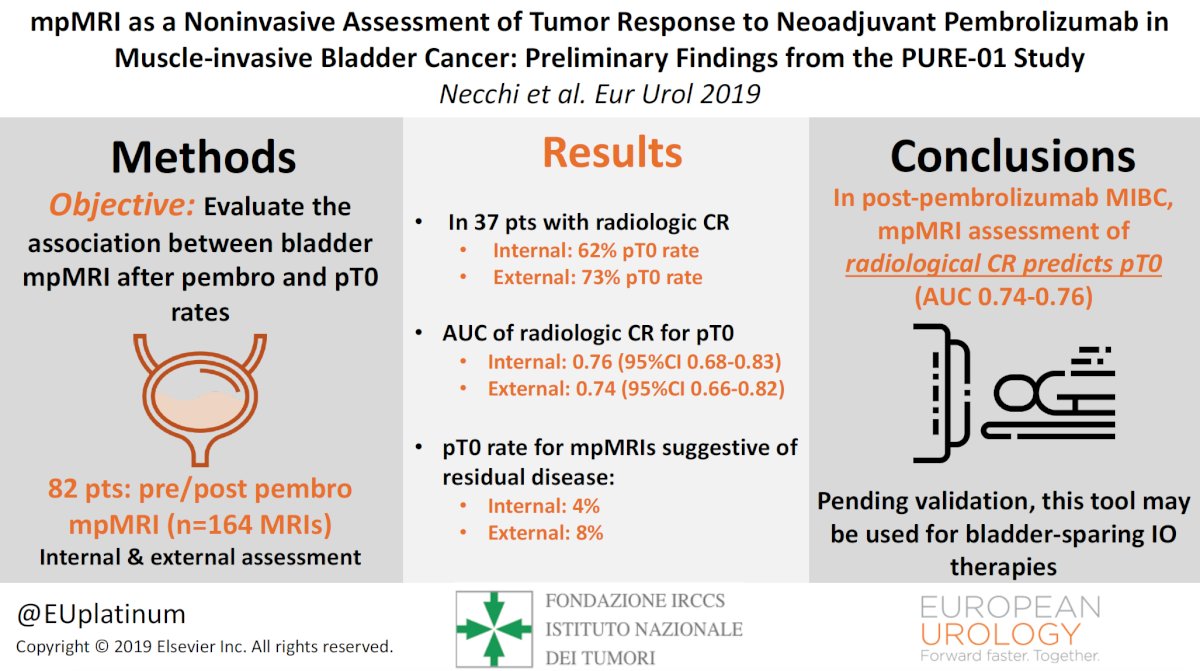
The following is a case example from PURE-01 of poor response after adjuvant pembrolizumab as assessed by mpMRI: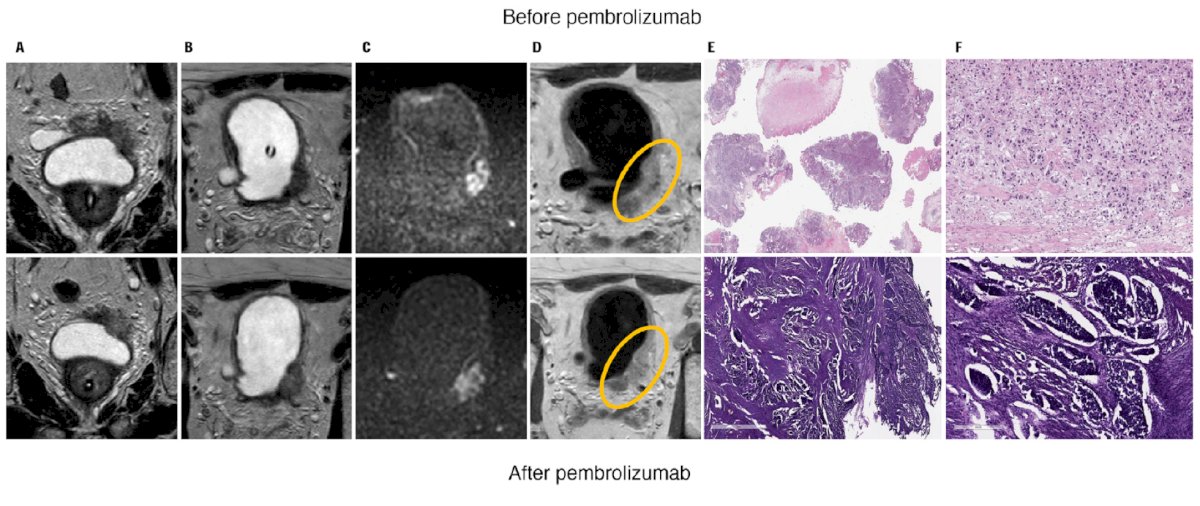
Another case shows a large baseline tumor burden, which is significantly reduced and seen clearly on mpMRI after adjuvant pembrolizumab: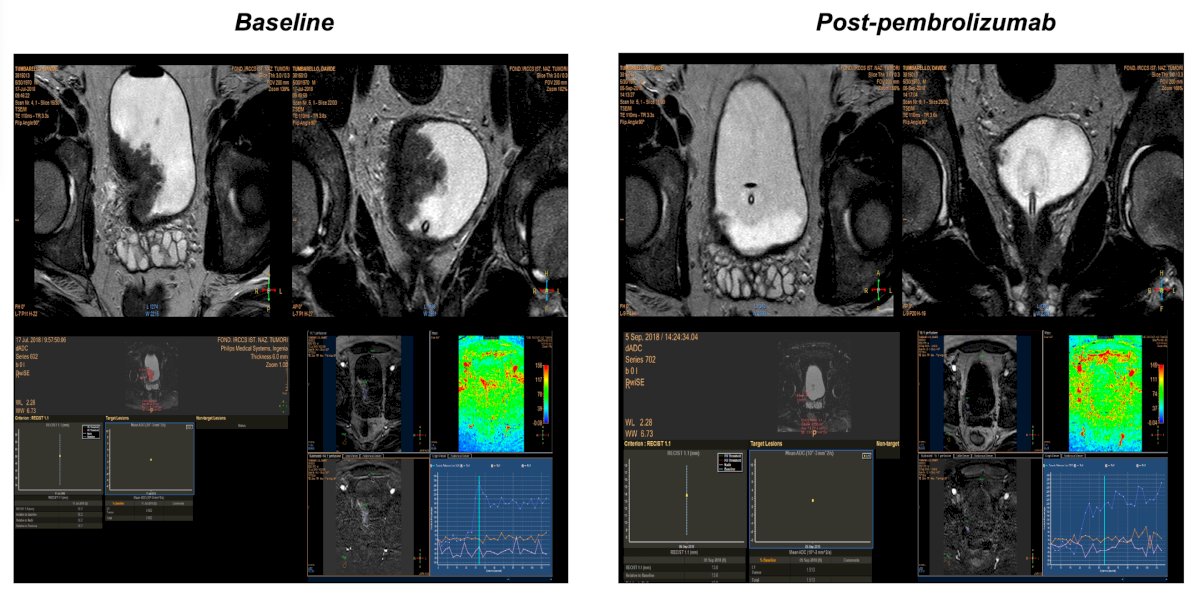
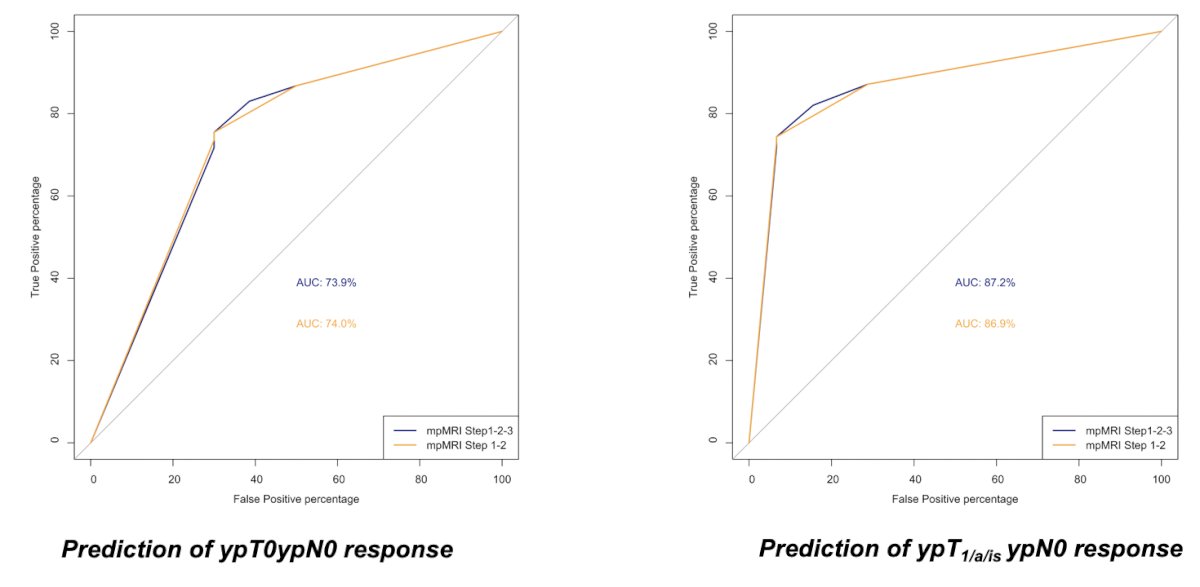
Dr. Necchi’s group led by Marco Bandini then asked whether biparametric MRI is as accurate as mpMRI. Using 143 patients in PURE-01,2 there were 123 patients with suitable paired imaging assessments before and after pembrolizumab tests (n = 246 mpMRI in total) that were analyzed in relation to the pathologic response. The area under the curve (AUC) of the combination of all sequences to predict ypT0ypN0 response was 0.74. By excluding dynamic contrast enhancement (DCE) assessment, the AUC was 0.74. When looking at ypT1/a/is ypN0 response, the AUC was 0.87 in both cases:
Thus, we may be able to avoid intravenous gadolinium contrast to personalize bladder-sparing strategies in radiologically complete responders. Is there a role for combined imaging modalities? And will differences in quantitative parameters define response? Dr. Necchi notes that bladder PET/MRI is being prospectively evaluated in the SOGUG-NEOWIN trial and should answer the aforementioned questions.
The new photon counting CT offers superior spatial resolution compared to MRI (0.169 mm versus 3 mm) with the capability to characterize transmural involvement. Spectral information with monoenergetic reconstruction (45 KeV) and iodine maps allows identification of focal areas of hyperenhancement suggestive for persistence of disease within areas of increased thickness for post-respective edema/inflammation. On the following images, focal areas of hyperenhancement with transmural involvement at monoenergetic reconstruction are clearly visible on the iodine map in a patient with ypT2 disease: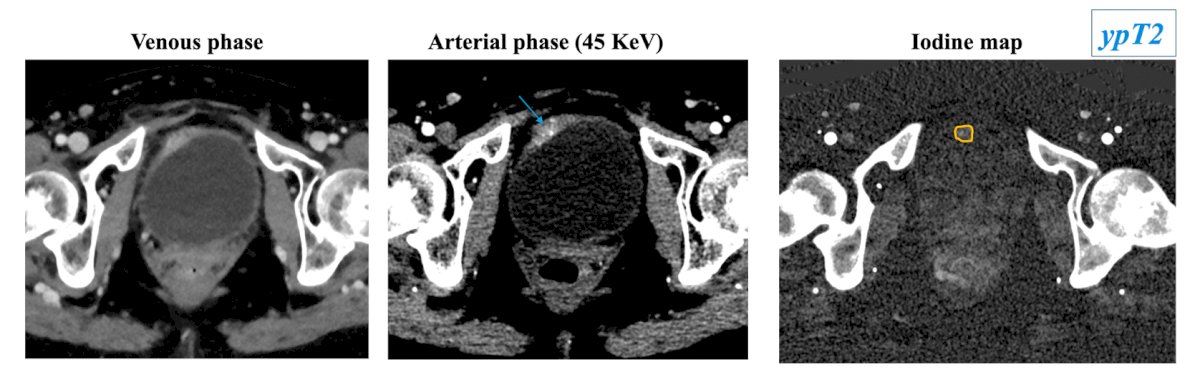
Dr. Necchi notes that tumor biomarkers (from TURBT) have shown incremental possibilities of achieving a ypTN response, however, none of them have provided reliable opportunities to identify the outlier responders. Conversely, clinical response has provided a close association with ypT0N0 response and may serve as a biomarker to orient personalized bladder-sparing strategies.
Dr. Necchi then focused on the new nacVI-RADS system, using VI-RADS for therapeutic response assessment. The nacVI-RADS workflow to assess the likelihood of complete response to therapy is highlighted in the following figures: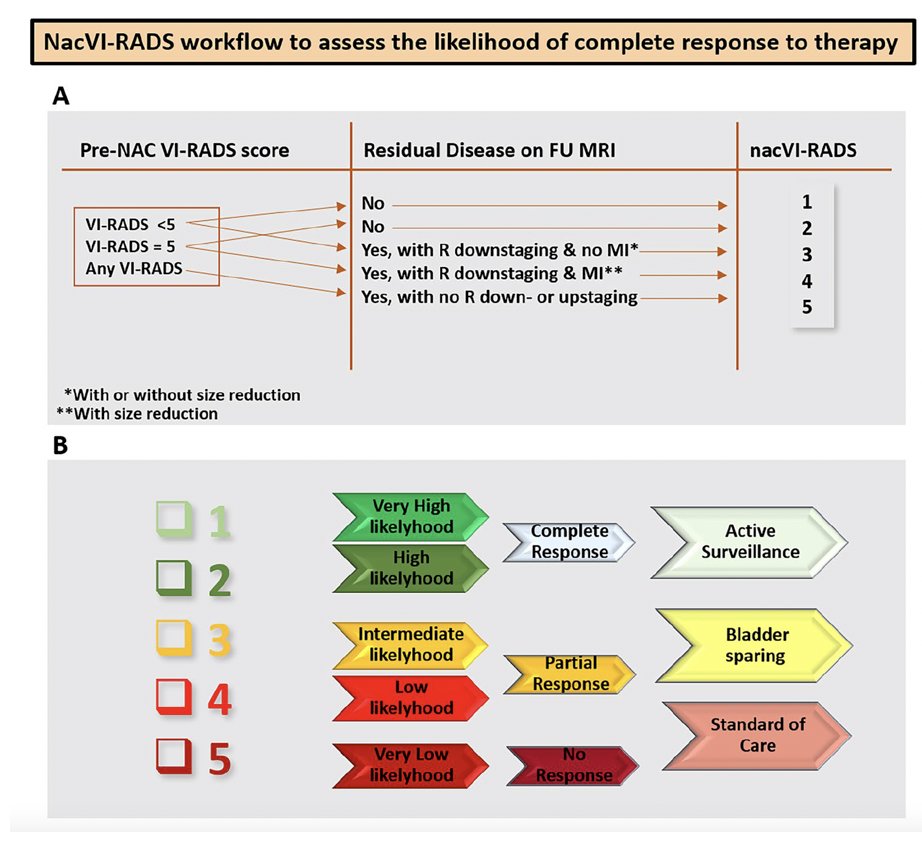
Dr. Necchi’s group recently looked at nacVI-RADS in the PURE-01 trial.3 In this study, among 220 MRI scans, they noted the importance of having a matched pre-post therapy assessment to evaluate a clinical response to treatment. Overall, 32.7% of patients with pre-pembrolizumab VIRADS 4-5 were downgraded to post-pembrolizumab VI-RADS 0-3. Additionally, they introduced the VI-RADS ‘0’ category to indicate no evidence of disease on MRI. On logistic regression analyses predicting pathological downstaging to ypT<=1N0, pre-VI-RADS 0-3 versus 4-5 was the strongest predictor on multivariable analyses (OR 3.98, 95% CI 1.32-12.76).
Highlighting work in progress using the PURE-01 dataset, Dr. Necchi notes that among patients that underwent cystectomy (standard reference), VI-RADS criteria (reviewed by 5 radiologists) had an accuracy of 76% for >ypT0 and an accuracy of 84% for >ypT1. Furthermore, only 4% of VI-RADS score 0 patients were ypT2 after radical cystectomy.
Dr. Necchi then discussed the reproducibility of VI-RADS findings across various neoadjuvant therapies, starting with the SURE-01 trial (sacituzumab govitecan x 4 cycles followed by radical cystectomy):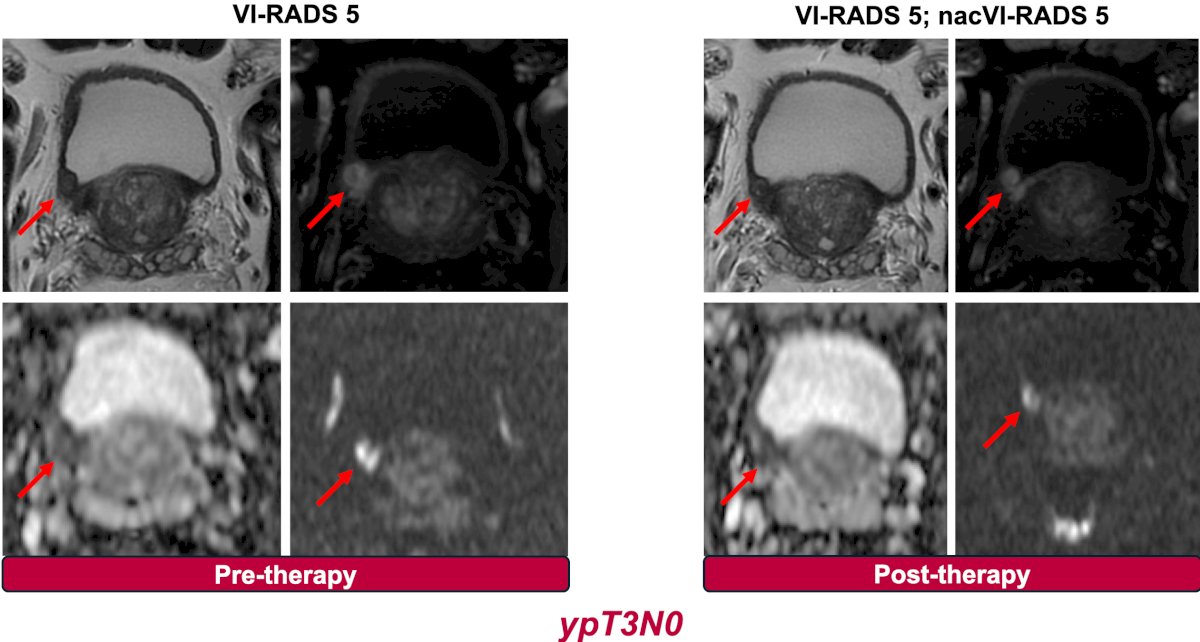
As well as the NURE-Combo trial (nivolumab + nap-paclitaxel x 4 cycles followed by radical cystectomy followed nivolumab x 13 cycles):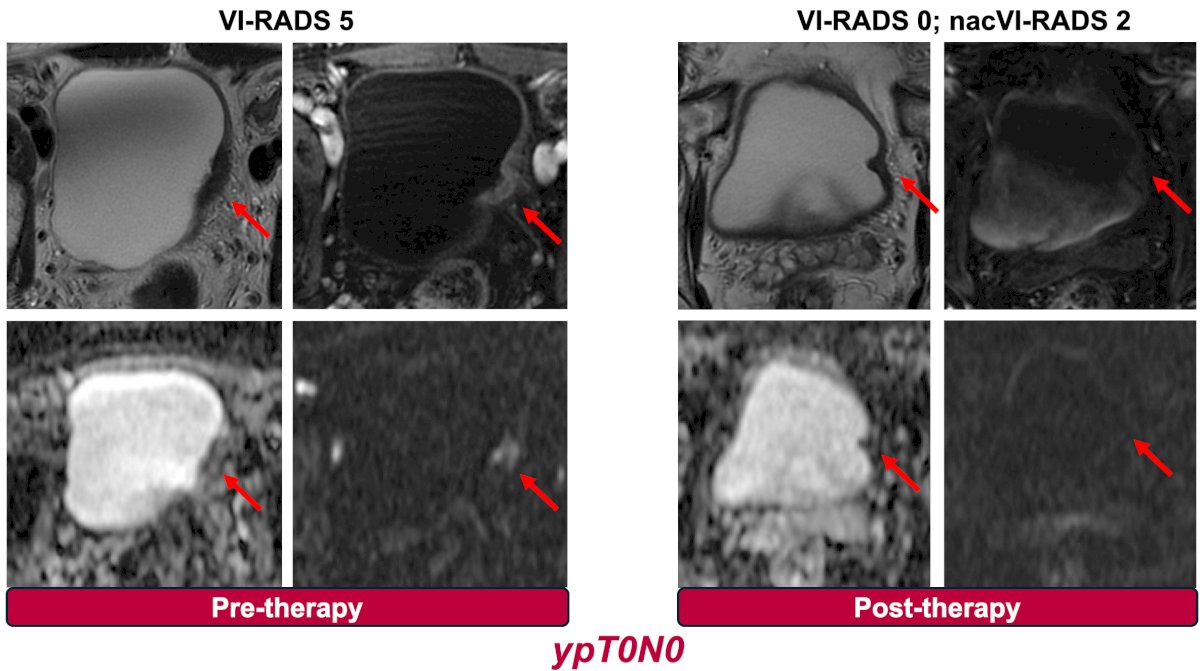
And the OPTIMUS trial (epacadostat followed by radical cystectomy):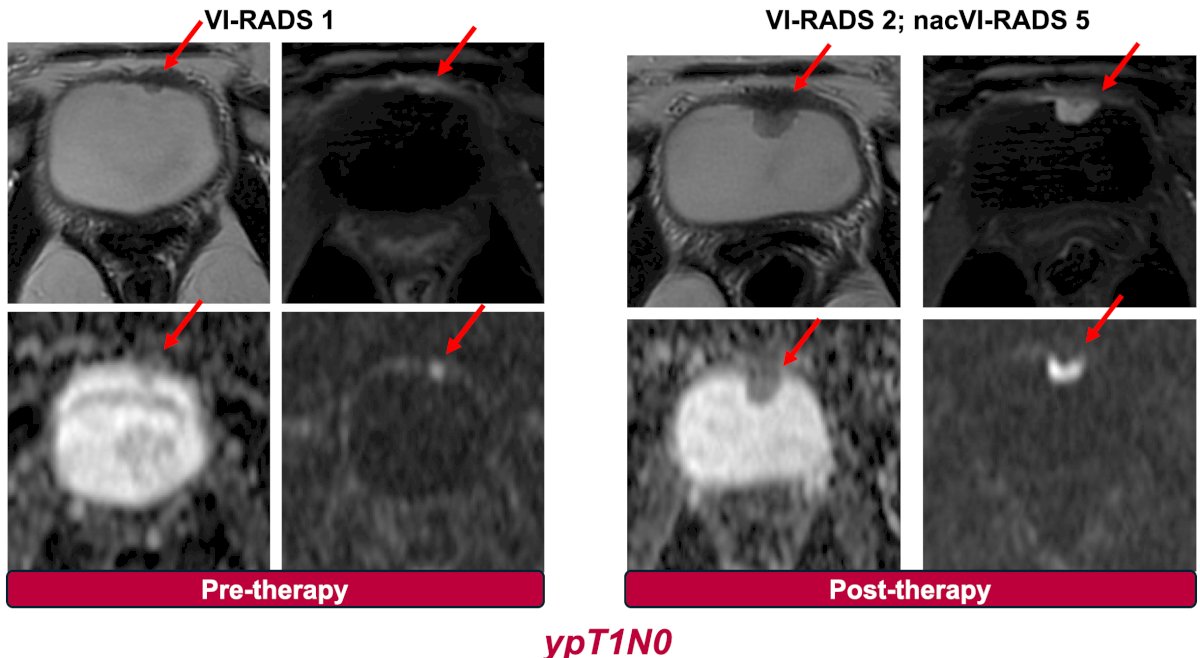
Dr. Necchi notes that outcomes of adjuvant pembrolizumab in the PURE-01 trial stratified by VI-RADS 0-3 vs 4-5 are similar to those of adjuvant nivolumab + gemcitabine + cisplatin in the HCRN GU 16-257 trial assessing VI-RADS 1-2 versus 3-5: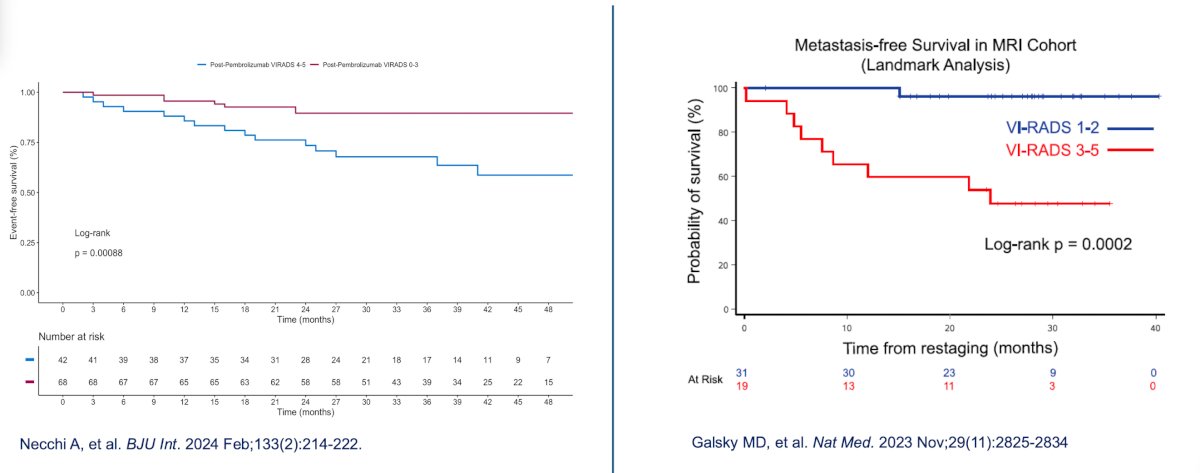
The utilization of excellent imaging allows a response-based flexibility, and the option of bladder preservation using systemic therapy. Cystectomy and radical radiotherapy are associated with quality of life implications, and some patients have a remarkable response to neoadjuvant systemic therapy. As such, there is data emerging for favorable outcomes with TURBT + chemotherapy in selected patients. However, two outstanding questions remain:
- Can deep responders to systemic treatment keep their bladders intact?
- Can the neoadjuvant window of opportunity result in a bladder-sparing opportunity?
Dr. Necchi concluded his presentation discussing the value of mpMRI in assisting decision-making in muscle-invasive bladder cancer with the following take-home points:
- Bladder MRI and VI-RADS use have shown incremental possibilities to better stage muscle invasive bladder cancer and predict complete response to neoadjuvant therapy. Despite level 1 evidence, MRI should be incorporated in modern management of patients with muscle-invasive bladder cancer.
- Composite models aimed to incorporate local response information (MRI, biopsy, urine, cytology) and liquid biopsy assessment (ctDNA) should be prospectively evaluated within trials.
- Uncertainties exist regarding the assessment and management of residual non-infiltrating disease (ypTa/T1/Tis)
- There seems to be consistency of the performance of VI-RADS assessment across the neoadjuvant therapy regimens.
- There is a need for a consensus/harmonization among investigators, patient advocates, regulatory agencies, etc on the complete response definition for the design of the next-generation bladder-saving trials
Presented by: Andrea Necchi, MD, Medical Oncologist, Professor of Oncology, Vita-Salute San Raffaele University, Chief of Genitourinary, Medical Oncology, Fondazione IRCCS Instituto Nazionale dei Tumori, IRCCS San Raffaele Hospital and Scientific Institute, Milan, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024
References:- Necchi A, Bandini M, Calareso G, et al. Multiparametric Magnetic Resonance Imaging as a Noninvasive Assessment of Tumor Response to Neoadjuvant Pembrolizumab in Muscle-invasive bladder cancer: Preliminary findings from the PURE-01 Study. Eur Urol. 2020 May;77(5):636-643.
- Bandini M, Calareso G, Raggi D, et al. The value of multiparametric magnetic resonance imaging sequence to assist in the decision-making of muscle-invasive bladder cancer. Eur Urol. 2021 Oct;4(5):829-833.
- Necchi A, Basile G, Gibb EA, et al. Vesical Imaging-Reporting and Data System use predicting the outcome of neoadjuvant pembrolizumab in muscle-invasive bladder cancer. BJU Int. 2024 Feb;133(2):214-222.


