(UroToday.com) The 2024 IBCN annual meeting included a session on novel therapies and outcome measures in clinical trials, featuring a presentation by Dr. James Sullivan discussing preclinical characterization and translation to the clinic of EG-70. EG-70 (detalimogene voraplasmid) is an investigational, non-viral gene therapy designed to elicit local stimulation of anti-tumor immune response in the bladder and drive durable efficacy in patients with BCG-unresponsive high-risk NMIBC while mitigating the risk of systemic toxicities from immune stimulation:
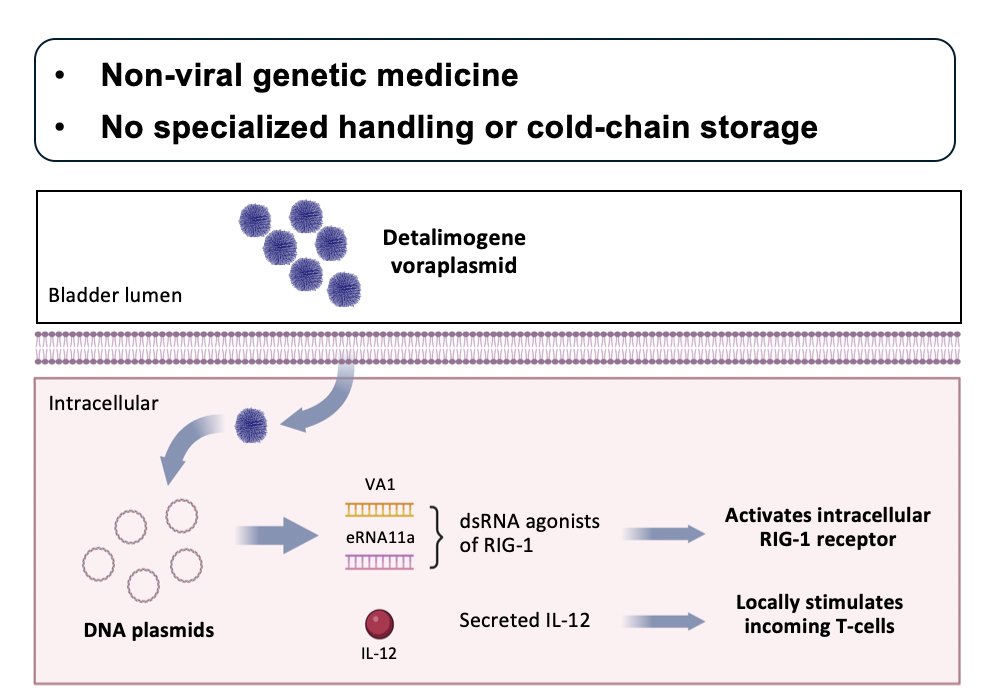
Mechanistically, there are two important points:
- Innate immune system activation with dual RIG-I agonists:
- Combination of NK cell stimulation and suppressor cell attenuation promotes tumor-killing
- Stimulates T-cell recruitment and neo-antigen presentation
- Adaptive immune system activation with secreted IL-12:
- T-cell-dependent cytokine response promotes tumor killing and immune memory
- Bladder-restricted production has potential to drive strong therapeutic effect while reducing potential for systemic adverse events.
At IBCN 2024, Dr. Sullivan and colleagues describe the latest preclinical data and phase 1 clinical study results.
From a preclinical perspective, a murine surrogate formulation of EG-70 (mEG-70) was administered locally to the bladder by intravesical instillations in C57BL/6 mice. Efficacy was assessed in a luciferase-expressing MB49 model of orthotopic bladder cancer. Following confirmation of cancer cell implantation, mice received two weekly intravesical instillations of mEG-70. Immune profiling was evaluated by assessing protein expression levels and immune cell compositions. In the orthotopic bladder cancer model, intravesical instillations of mEG-70 caused striking remodeling of the tumor microenvironment from an immunosuppressive to a pro-inflammatory phenotype, demonstrated by a significant decrease in myeloid cells and IL-4 cytokine levels, an increase in NK-cells and T-cells, and an increase in pro-inflammatory cytokines in tumor tissue: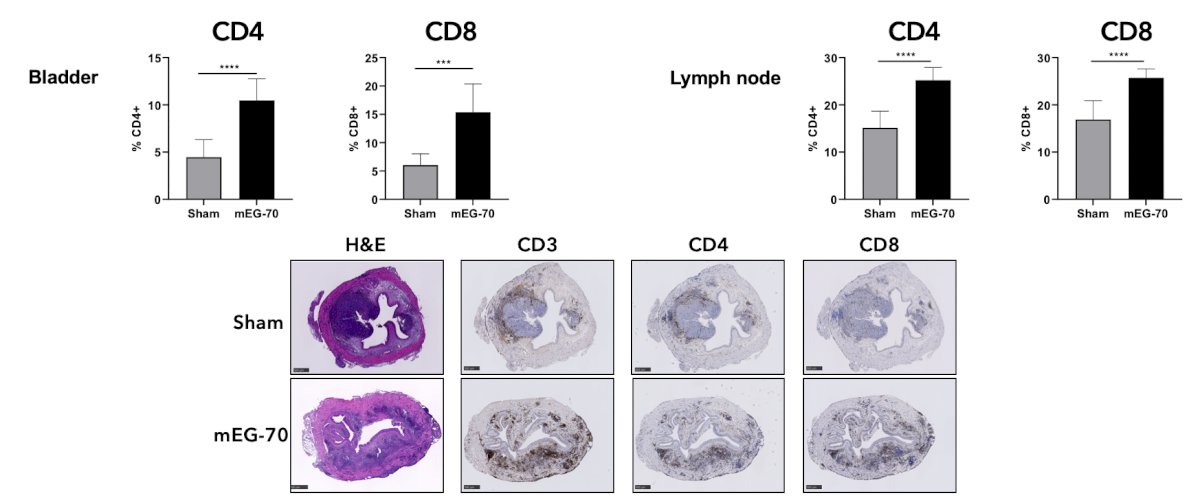
Administration of mEG-70 was associated with marked reduction in tumor burden and significant improvement of survival in murine xenografts: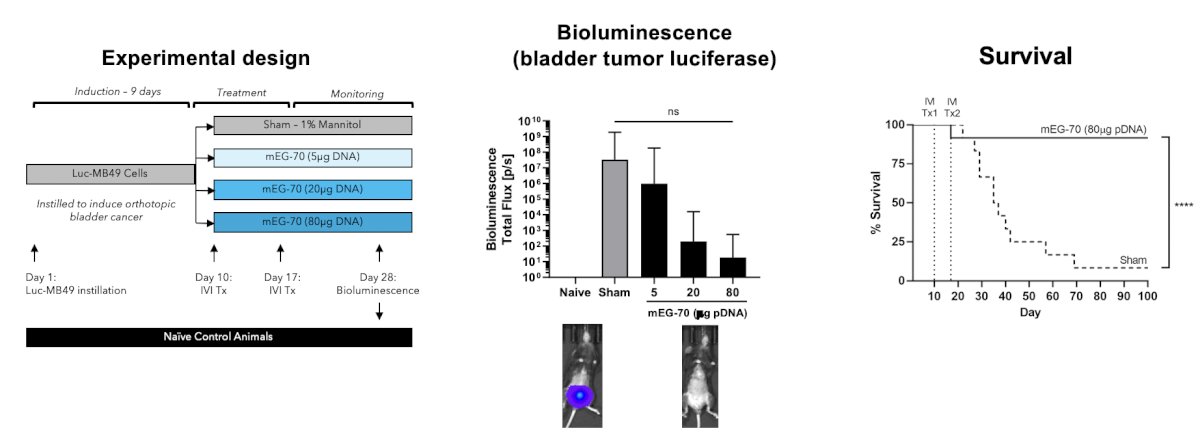
After flank implantation, the anti-tumor response was largely immune-mediated, with durable clearance of tumors and protection against subsequent tumorigenic re-challenge, both locally in the bladder as well as distally: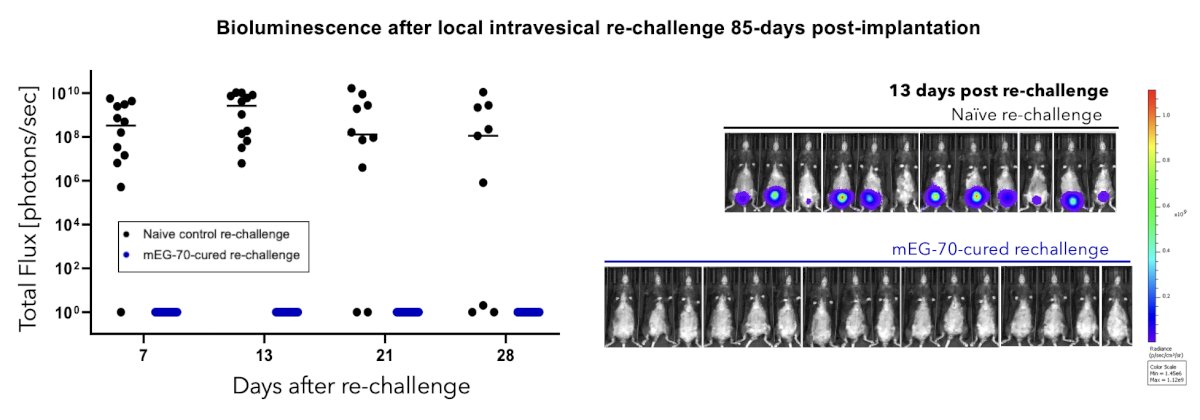
From a clinical perspective, EG-70 was assessed in a phase 1/2 study in 24 patients with high-risk BCG-unresponsive NMIBC with CIS (LEGEND; NCT04752722). EG-70 dosing was 2 or 4 doses every 12-week cycle, with a 3+3 dose escalation trial design. The primary endpoint for LEGEND was safety and the secondary endpoint were efficacy at 3 months.
Intravesical instillations of EG-70 was well-tolerated in patients with high-risk BCG-unresponsive NMIBC with CIS across all dose levels, with an overall complete response rate of 73%: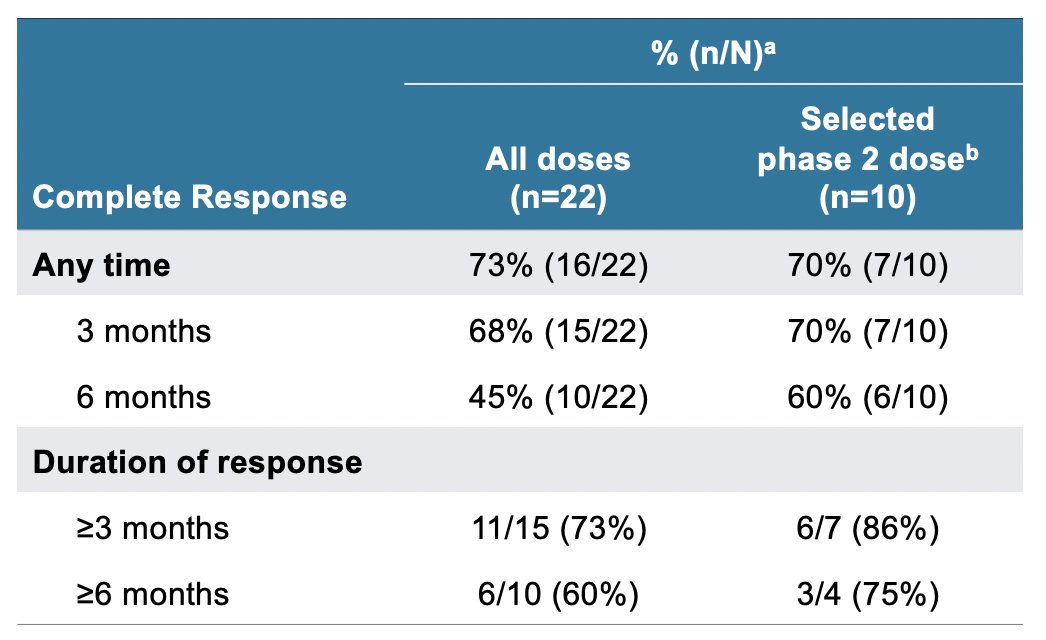
Any grade treatment-related adverse events occurred in 54.2% of patients, with no grade 4/5 events: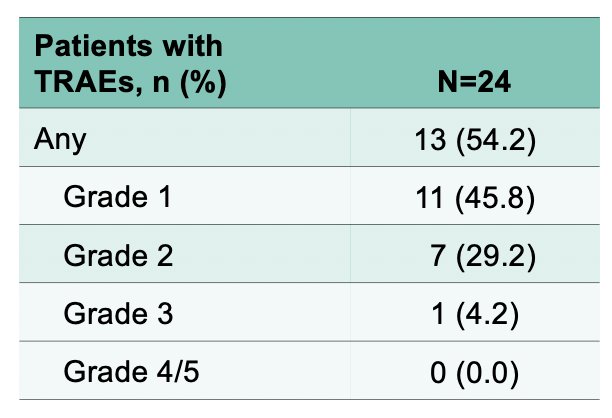
The most common treatment-related adverse events included hematuria, urinary tract infection, and micturition urgency: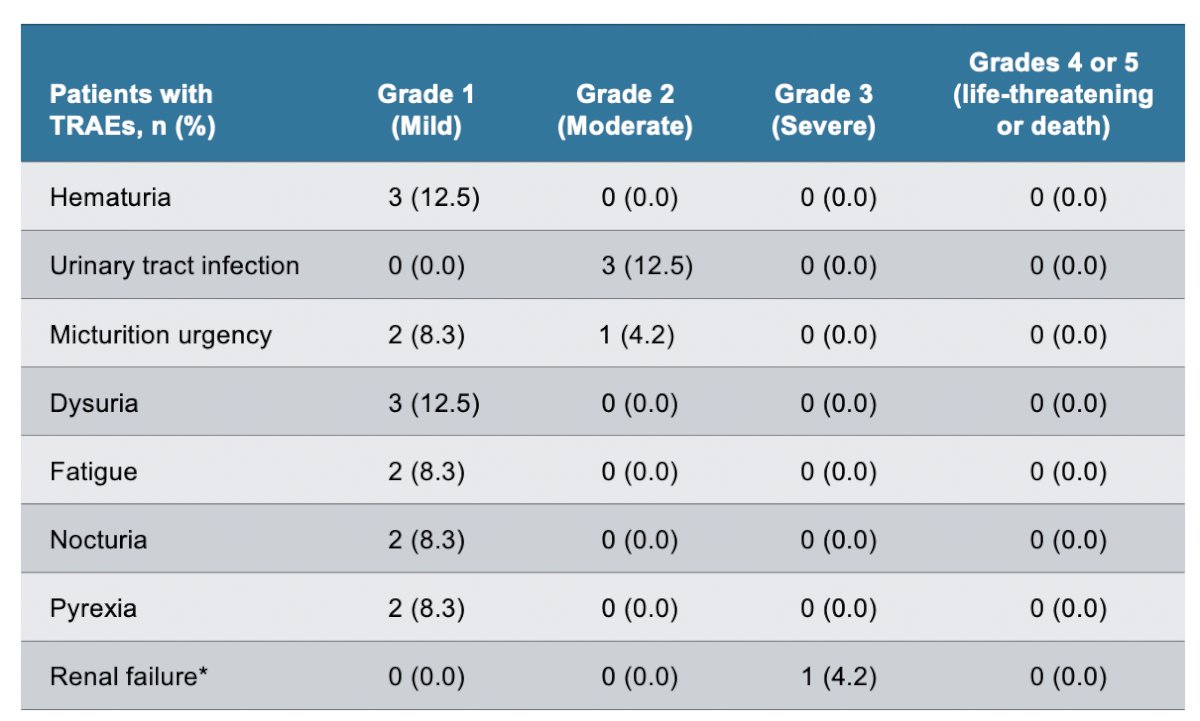
Dr. Sullivan concluded his presentation discussing preclinical characterization and translation to the clinic of EG-70, a novel, non-viral, intravesical gene therapy with the following take-home points:
- Preclinical data indicate that mEG-70 treatment results in robust activation of both innate and adaptive immune responses
- In tumor-bearing animals, mEG-70 remodels the tumor microenvironment resulting in clearance of existing tumors with a durable, memory-mediated protective effect.
- Interim data from the phase 1 portion of LEGEND suggest a promising safety, tolerability, and efficacy profile.
- Overall, 73% of patients achieved a complete response at any time. At the dose selected for phase 2, complete response rates were 70% at 3 months and 60% at 6 months.
- Treatment-related adverse events reported to date are mostly grade 1/2 and consistent with catheterization and intravesical administration.
Presented by: James Sullivan, PhD, Chief Scientific Officer, enGene Inc, Waltham, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024


