(UroToday.com) The 2024 IBCN annual meeting included a session on the current status and future directions of antibody drug conjugates, featuring a presentation by Dr. Srikala Sridhar discussing antibody drug conjugate toxicity, management and clinical trial updates. The clinical trial updates portion of Dr. Sridhar’s talk was given on behalf of Dr. Matt Milowsky who was unable to attend the meeting. Bladder cancer is the 9th most common cancer in the world, with >600,000 new cases worldwide. Patients present with an average age at diagnosis of 73 years, men are more frequently affected than women (3:1), with the key risk factor being smoking. The main histology is urothelial (pure or mixed), with some patients having variant histology. Depth of invasion into the bladder wall is important, and metastatic disease remains incurable. Until recently, life expectancy for metastatic disease was only 14 months, however new advances, including immune checkpoint inhibitors and antibody drug conjugates, have led to a nearly doubling of survival, thus it is a very exciting time in the field.
Dr. Sridhar notes that several antibody drug conjugates are already approved, with many others in development. The focus of this talk will be on antibody drug conjugates targeting Nectin, Trop2, HER2, and EGFR/HER3. Enfortumab vedotin is a fully humanized monoclonal antibody against nectin-4, and is conjugated with microtubule-disrupting agent, monomethyl auristatin E (MMAE), by a protease-cleavable linker:
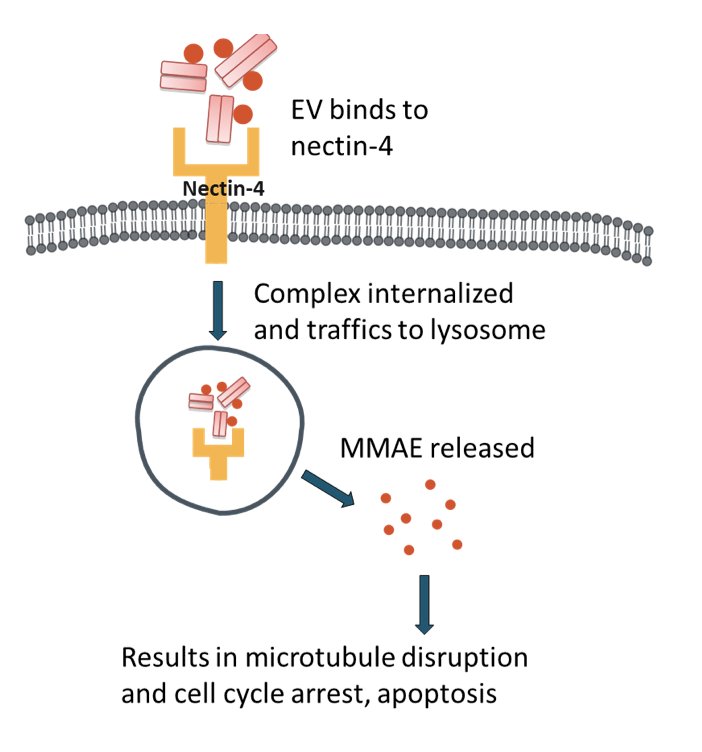
Nectin-4 is a transmembrane cell adhesion molecule that is highly expressed in 97% of metastatic urothelial carcinoma patient samples. In 2021, EV-301 was published assessing enfortumab vedotin in pre-treated locally advanced and metastatic urothelial carcinoma:1
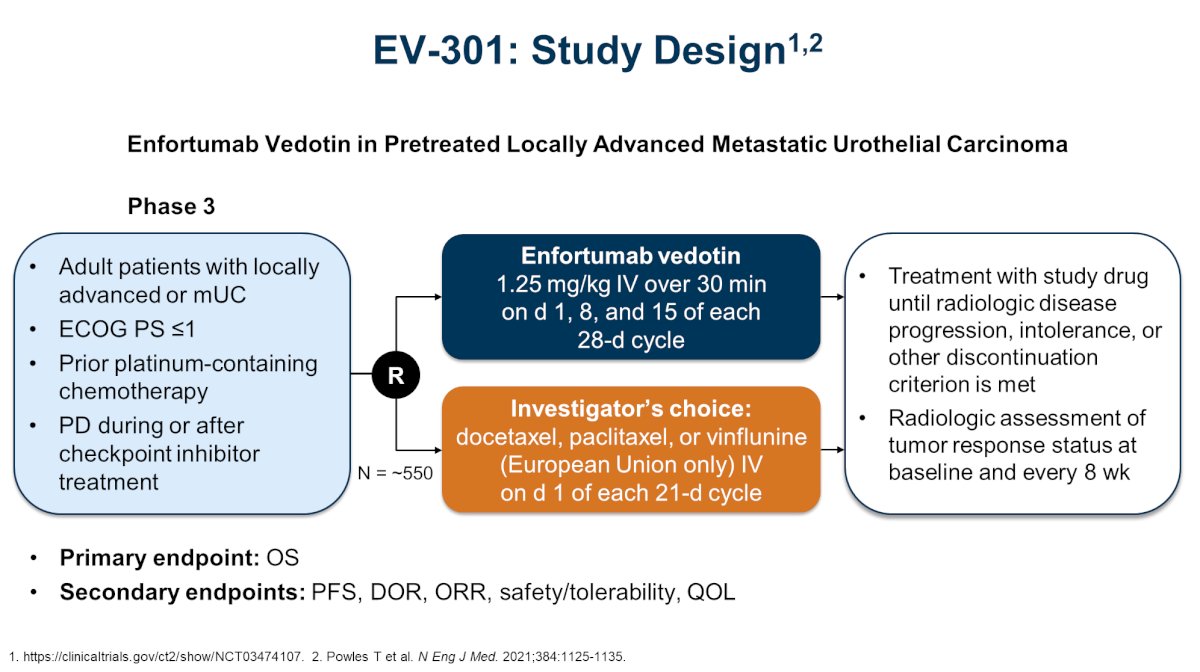
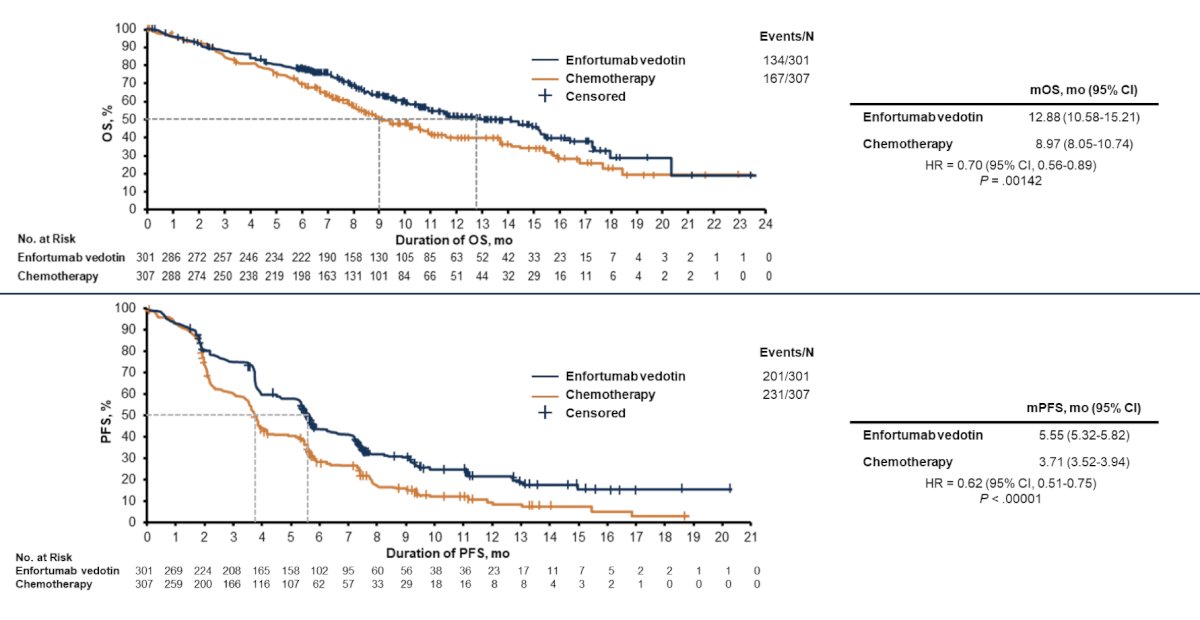
With regards to efficacy, objective response rates were higher with enfortumab vedotin versus chemotherapy (41% vs 18%, p < 0.001), and progression free survival was 5.55 vs 3.71 months (HR 0.62, 95% CI 0.51-0.75). Additionally, there was an overall survival advantage for enfortumab vedotin versus chemotherapy (12.88 months versus 8.97; HR 0.70, 95% CI 0.56-0.89):
Based on these outcomes, enfortumab vedotin was FDA approved in the post-chemotherapy/post-immune checkpoint inhibitor setting. Dr. Sridhar notes that with regards to adverse events, most were mild to moderate severity, and patients need to be encouraged to report side effects so they can be managed with dose delays, reductions, and steroids. Enfortumab vedotin treatment related adverse events of special interest included:
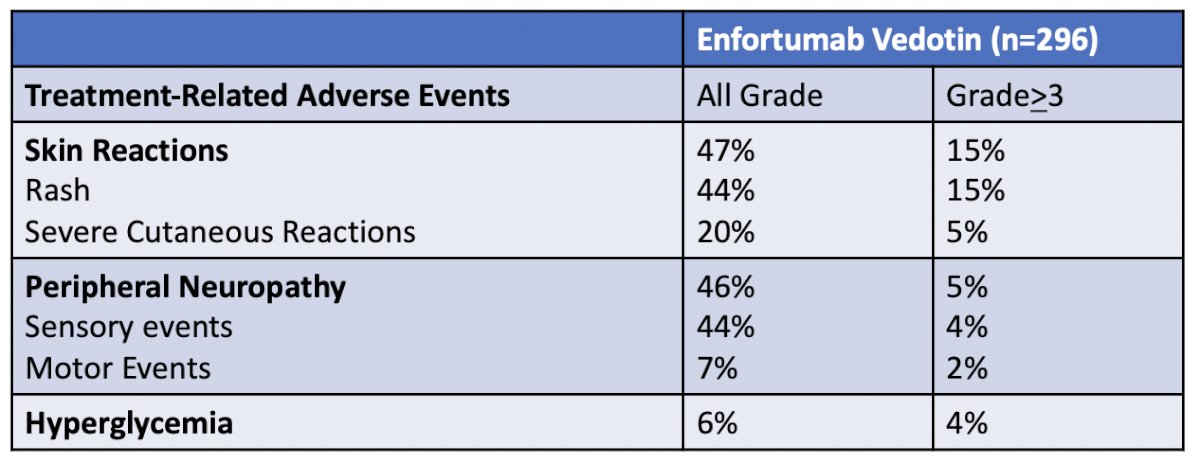
Skin toxicity is common for enfortumab vedotin, occurs early, and varies in presentation. This includes maculopapular rash, blisters, dry skin, hyperpigmentation, scaly papules, and rarely life-threatening manifestations like Stevens-Johnson Syndrome, and Toxic Epidermal Necrolysis. Management of low-grade toxicity is supportive with topical corticosteroids and antihistamines, with grade ≥ 3 events require treatment interruption, oral corticosteroids, and dermatologic consultation. Of note, treatment can resume with dose reduction for grade 3 events that have improved to grade ≤ 1. As follows is a summary of potential dermatologic issues potentially associated with enfortumab vedotin:
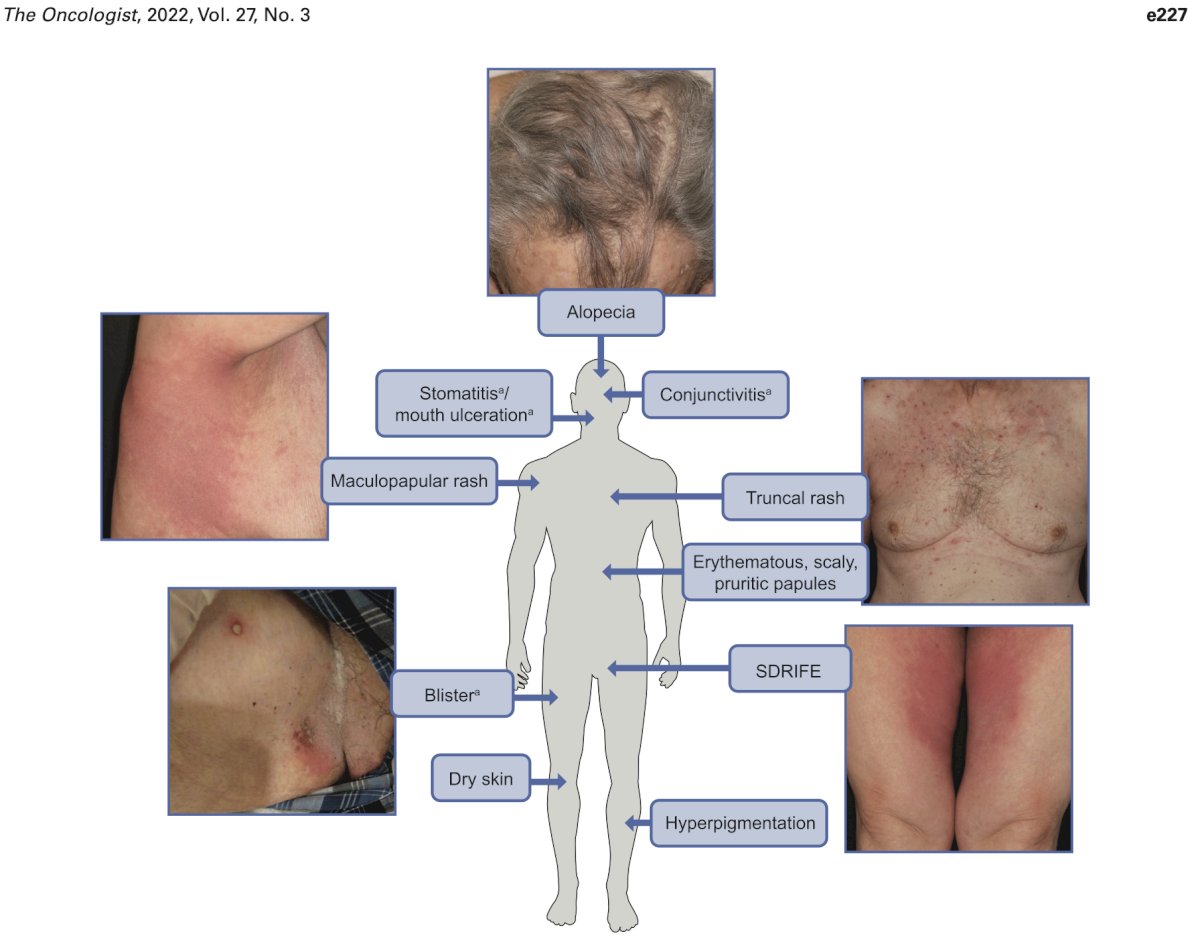
It is important to emphasize that skin toxicity occurs due to nectin expression in the epidermis, epithelium of sweat glands, and hair follicles. So, could skin toxicity be a biomarker for response? Retrospective work assessing 78 metastatic urothelial carcinoma patients found 42 (53.8%) experienced enfortumab-related cutaneous toxicity that appeared early. Cutaneous toxicity correlated with significantly improved overall survival (HR 0.48, 95% CI 0.25, 0.90), objective response rate was 68.3% versus 20.7% (p = 0.0033), and disease control rate was 82.9% versus 48.3% (p = 0.0122). The median progression free survival was numerically longer in the cutaneous toxicity group (6.3 versus 1.7 months), but not statistically significant on multivariable analysis.
With respect to enfortumab vedotin and neuropathy, this occurs later than skin toxicity. From a neurologic standpoint, typically sensory is worse than motor neuropathy, and lower extremities are worse than upper extremities, with the sural nerve the most commonly affected. Putative pre-treatment factors associated with a higher risk include higher pre-treatment albumin levels and pre-existing neuropathy. Importantly, the association between pre-existing DM and enfortumab vedotin-induced peripheral neuropathy requires further elucidation. Initial results suggest closer monitoring, and earlier intervention to mitigate enfortumab vedotin-induced peripheral neuropathy may be warranted for certain patients starting enfortumab vedotin. Dr. Sridhar notes that enfortumab vedotin-induced peripheral neuropathy is a frequent dose-limiting toxicity that can affect cumulative therapeutic exposure, and thus treatment outcomes, while also negatively impacting quality of life. Prospective studies to identify and validate risk factors for enfortumab vedotin-induced peripheral neuropathy are critical for optimizing outcomes in patients with advanced urothelial carcinoma
Potential mechanisms for enfortumab vedotin peripheral neuropathy include:
- Target-dependent uptake of the antibody drug conjugates
- Non-specific uptake of antibody drug conjugates by healthy cells
- Premature MMAE release during circulation
- The bystander effects of MMAE-based antibody drug conjugates
In the peripheral nerve, MMAE binds to binding sites along the length of microtubules and disrupts the microtubules network, resulting in the onset of peripheral neuropathy. Schwann cells become activated and produce damage-associated molecular patterns (DAMPs), which in turn attract circulating leukocytes. This promotes the release of various pro-inflammatory cytokines, including IL-1β, TNFα, CXCL1, CXCL12, etc. These cytokines trigger neuroinflammation and convey nociceptive information to DRG neurons, leading to the perception of pain:
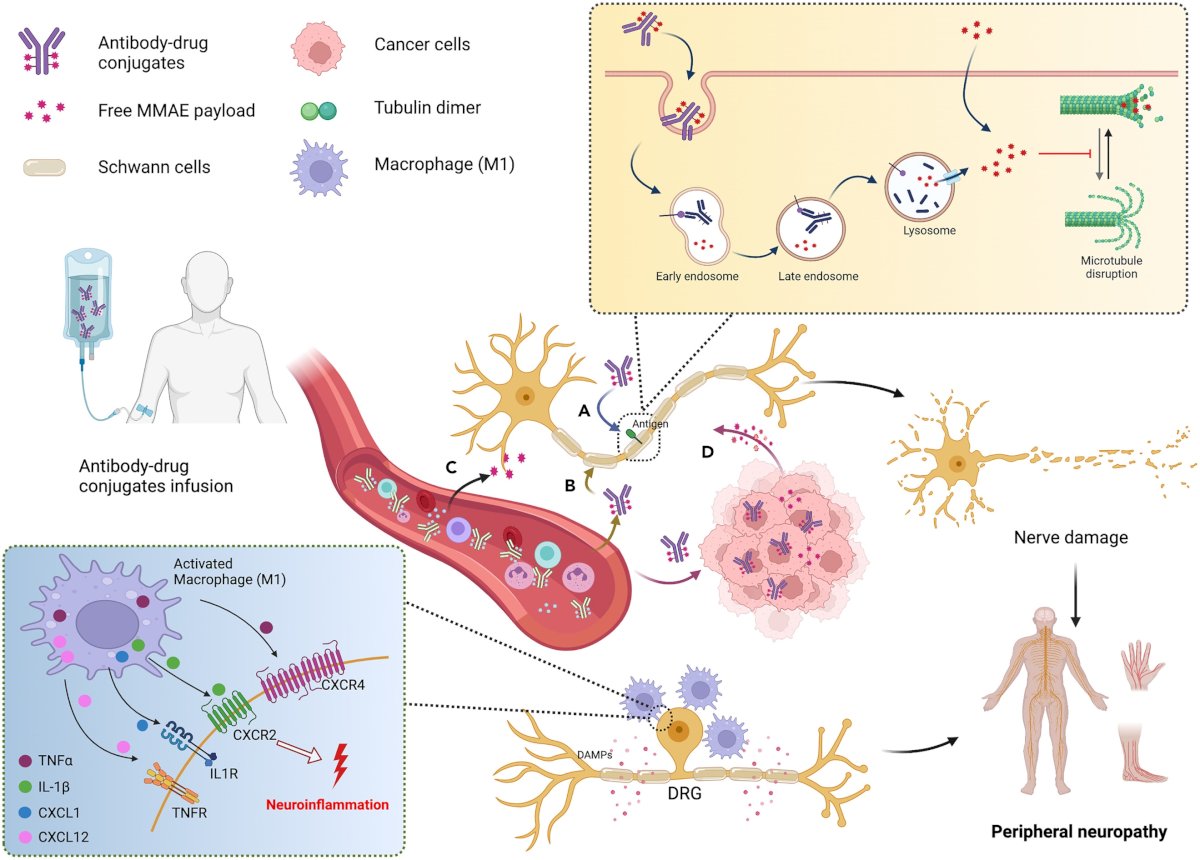
The next potential side effect of enfortumab vedotin is severe hyperglycemia, which is an unexpected and potentially fatal side effect, with an unclear mechanism of action. One hypothesis is that enfortumab vedotin directly blocks the insulin receptor, which would explain both the insulin resistance and diabetic ketoacidosis. Enfortumab vedotin has a half-life of 3.6 days, and in this case, higher therapeutic levels of enfortumab vedotin correlated with insulin resistance. Thus, Dr. Sridhar notes that blood sugar levels should be checked at each cycle of treatment, and patients need to be questioned about signs or symptoms of hyperglycemia.
Dr. Sridhar then discussed enfortumab vedotin + pembrolizumab which was initially presented in 2019 as part of the EV-103 trial, assessing the combination in locally advanced or metastatic urothelial carcinoma:
The confirmed objective response rate was 32% (95% CI 55.7 – 83.6). EV-103 also had Cohort K, which assessed enfortumab vedotin + pembrolizumab versus enfortumab vedotin alone. In the combination arm, 85.7% (41/49) of responses achieved at first assessment (9 ± 1 week), confirmed objective response rates comparable across subgroups, and 53.8% (7/13) of confirmed objective response rates were achieved in patients with liver metastases. The activity in the enfortumab vedotin arm as consistent with findings observed in second-line locally advanced or metastatic urothelial carcinoma:
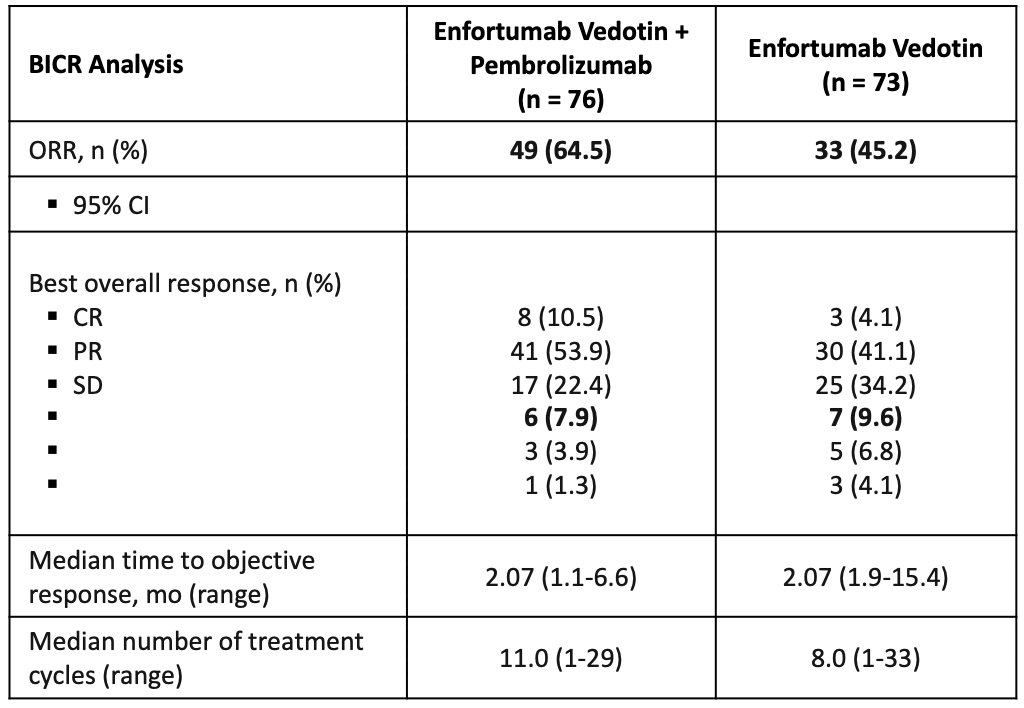
Thus, enfortumab vedotin + pembrolizumab initially received FDA approval in cisplatin-ineligible first-line metastatic urothelial carcinoma.
Next, Dr. Sridhar discussed the landmark trial presented at ESMO 2023 and subsequently published in The New England Journal of Medicine, the EV-302/KEYNOTE-A39 trial.2 Patients in this trial were randomized 1:1 to either enfortumab vedotin + pembrolizumab (n = 442) or gemcitabine + cisplatin/carboplatin (n = 444) for a maximum of 6 cycles. The dual primary endpoints were progression-free survival, assessed via blinded independent central review and overall survival. The study design is as follows:
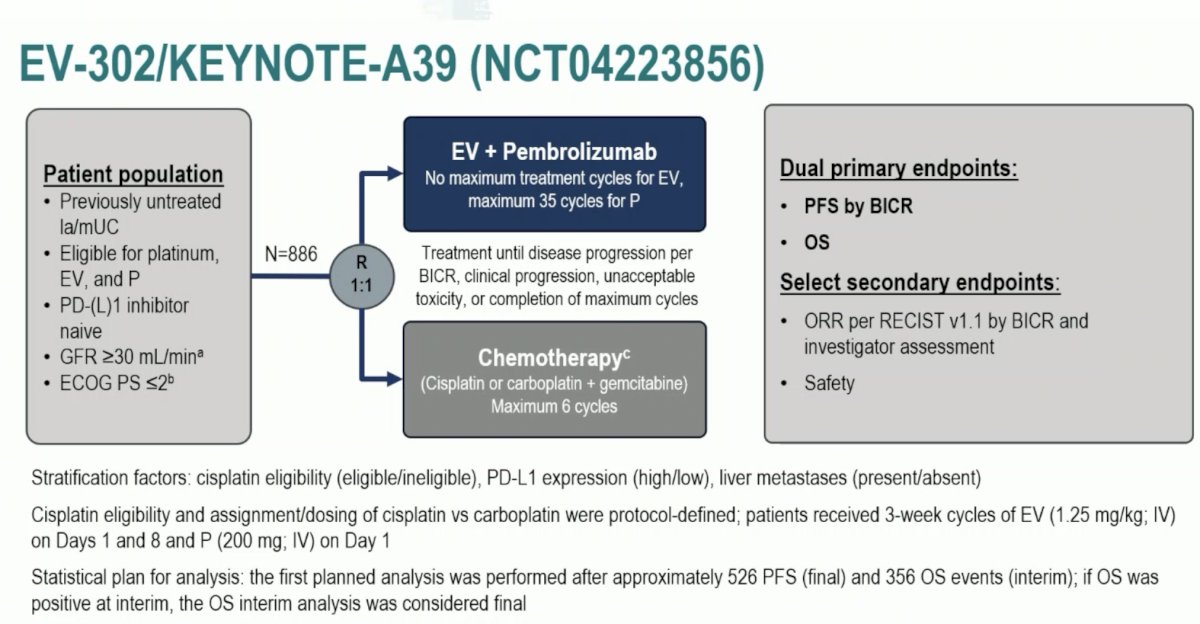
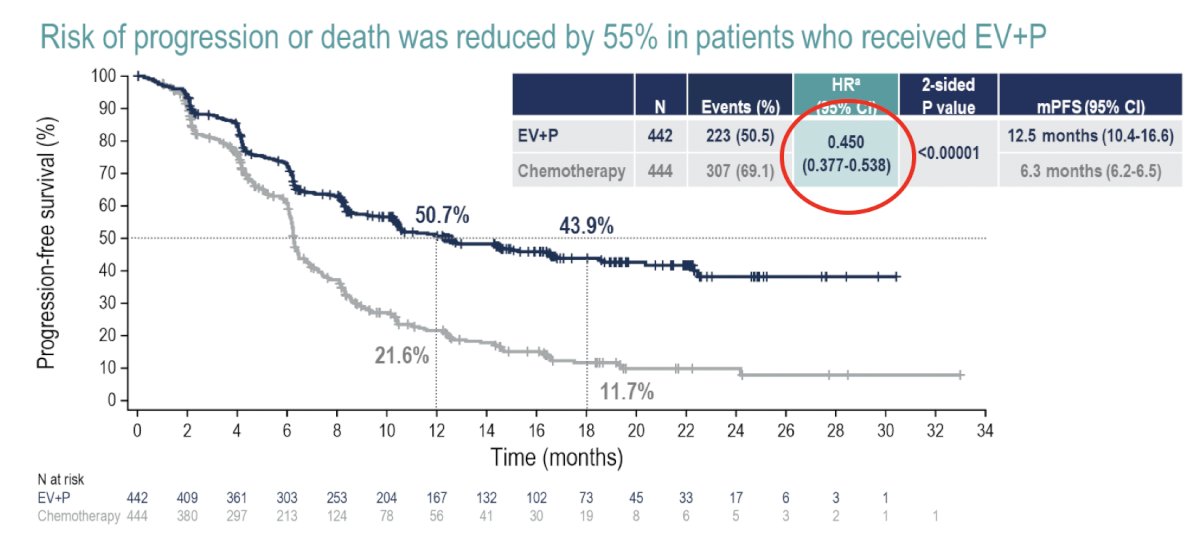
Compared to platinum-based chemotherapy, enfortumab vedotin + pembrolizumab prolonged progression-free survival from a median of 6.3 months to 12.5 months (HR: 0.45, 95% CI: 0.38 – 0.45, p<0.001):
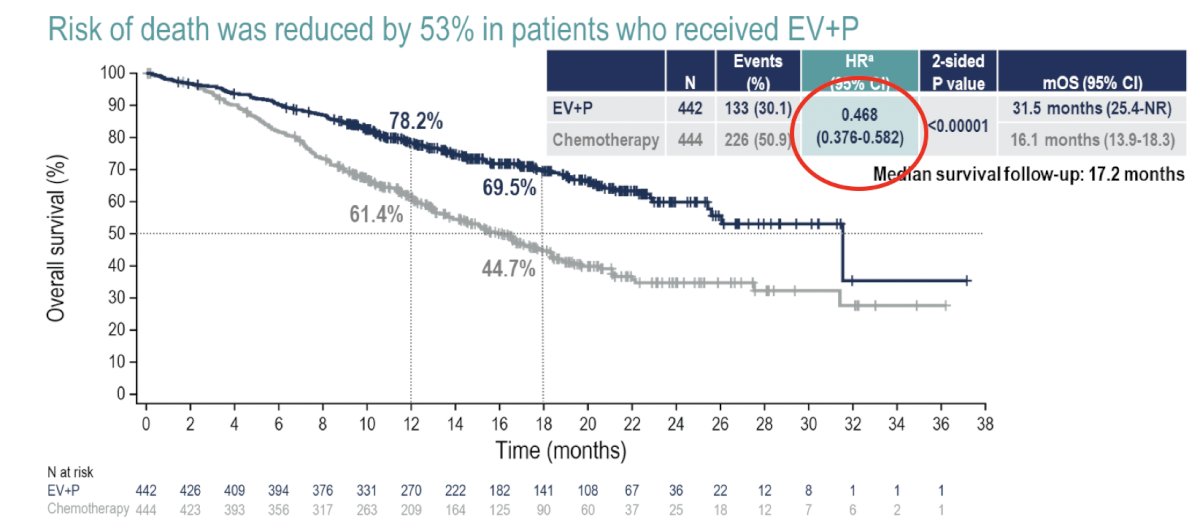
Overall survival was nearly doubled in the enfortumab vedotin + pembrolizumab arm, with median survivals of 31.5 and 16.1 months, respectively (HR 0.47, 95% CI 0.38 – 0.58,). These survival benefits were observed despite a higher proportion of patients in the chemotherapy arm receiving a subsequent systemic therapy (66.2% versus 28.9%):
Treatment-related adverse events were consistent with the known safety profiles of each of the drugs. Serious treatment-related adverse events occurred in 27.7% of patients in the enfortumab vedotin + pembrolizumab arm, compared to 19.6% with chemotherapy. Treatment-related adverse events leading to death occurred in 4 patients (0.9%) in each arm. Overall, grade 3+ adverse events occurred in 56% and 70% of patients in the enfortumab vedotin + pembrolizumab and chemotherapy arms, respectively. The most common enfortumab vedotin treatment-related adverse events were skin reactions, peripheral neuropathy, ocular disorders, and hyperglycemia:
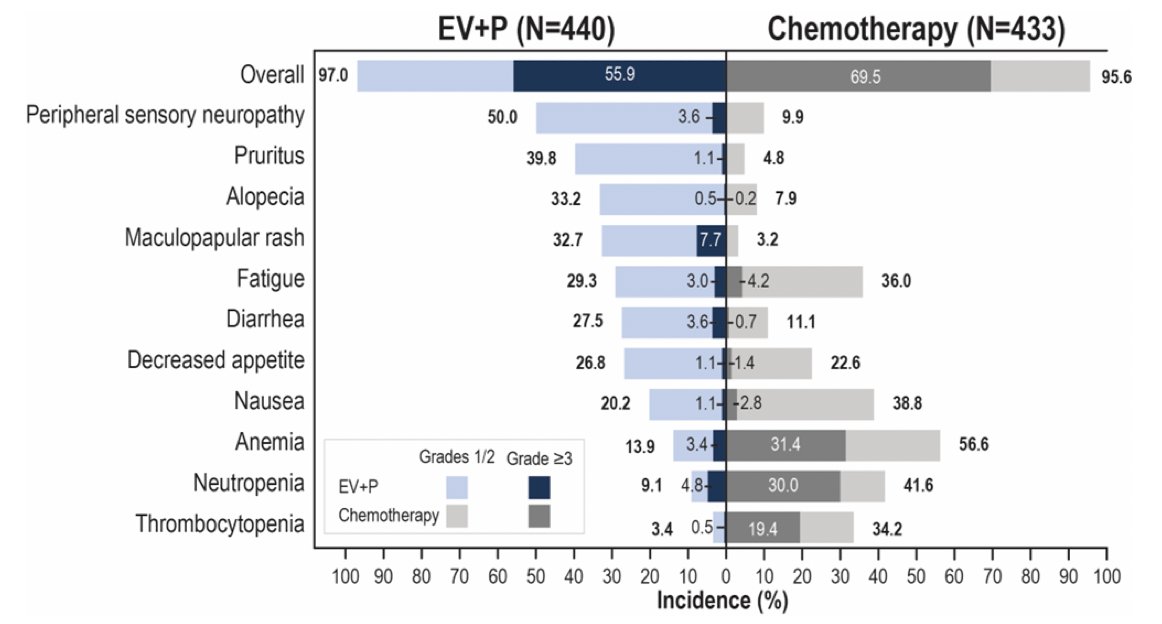
In EV-302, enfortumab vedotin treatment-related adverse events of special interest were primarily low grade:
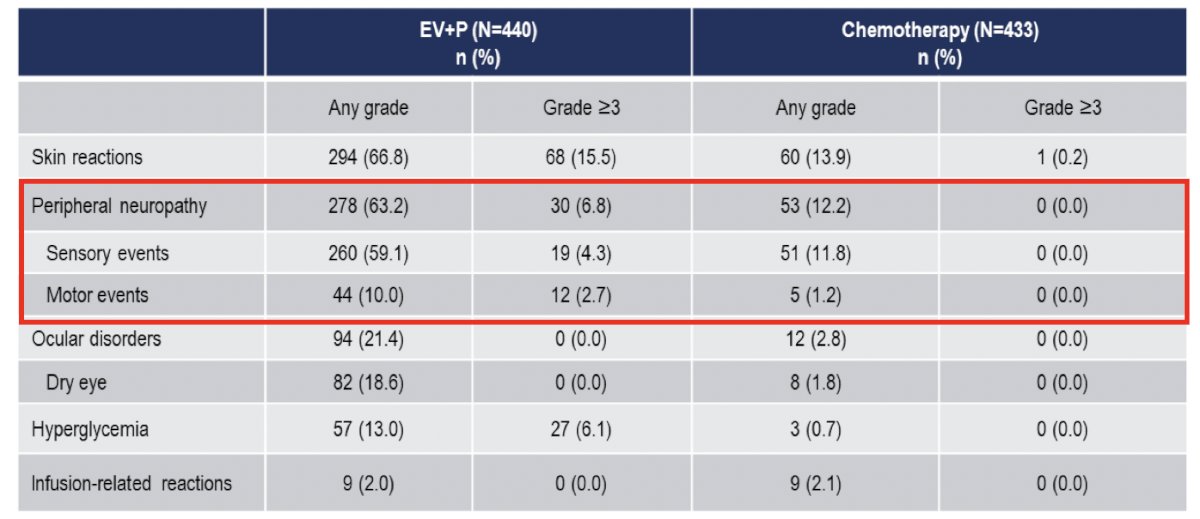
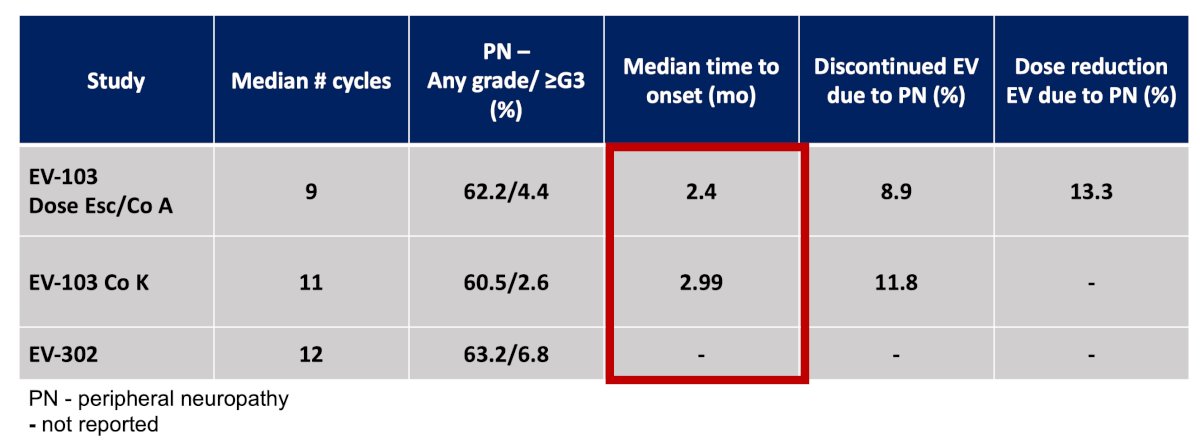
Peripheral neuropathy with enfortumab vedotin + pembrolizumab has been assessed several studies, noting that onset of neuropathy is generally quite early:
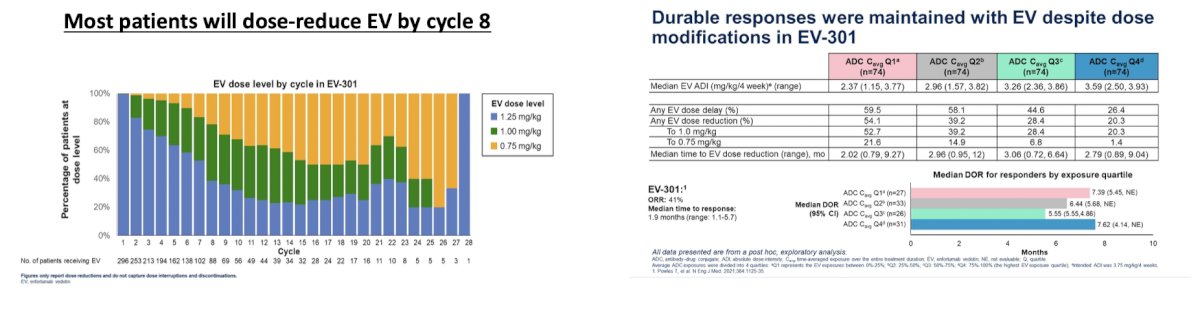
Dr. Sridhar highlighted that the combination of enfortumab vedotin + pembrolizumab is now listed as a first-line option for metastatic urothelial carcinoma for those that are eligible. At ASCO 2024, Dr. Petrylak assessed the impact of exposure on outcomes with enfortumab vedotin, emphasizing that most patients will dose-reduce by cycle 8, and durable responses can be maintained with enfortumab vedotin despite modifications in EV-301:
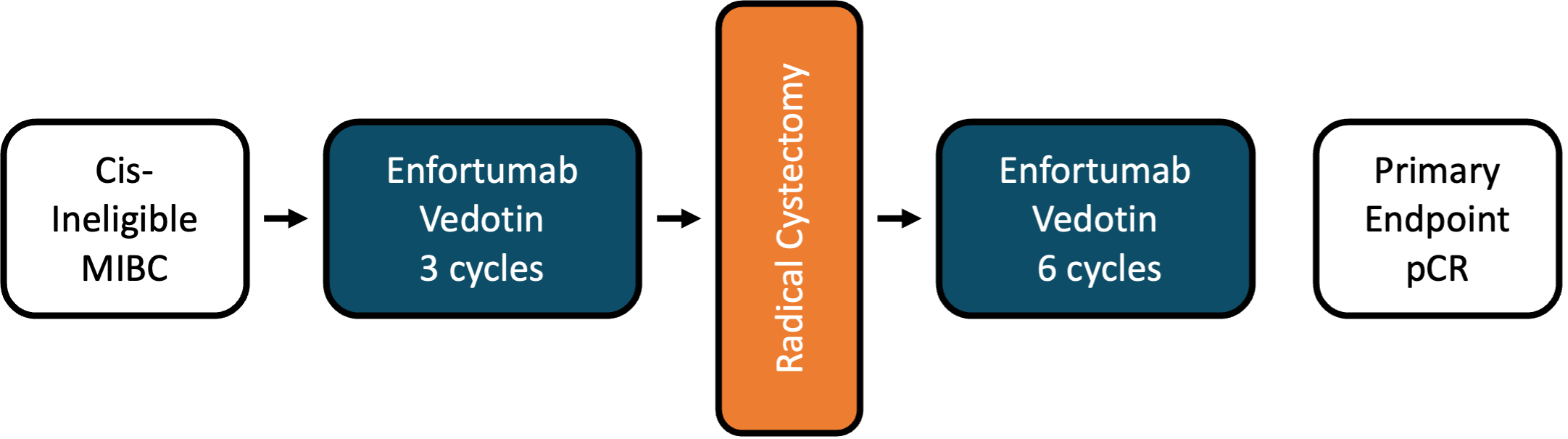
The EV-103 cohort H trial assessed neoadjuvant enfortumab vedotin monotherapy in cisplatin-ineligible patients with muscle invasive bladder cancer. This trial noted a pathologic complete response rate of 36.4% and an event free survival rate at 12 months of 76.4% (95% CI 52.2-89.4). Adverse events in cohort H were comparable to previous enfortumab vedotin studies. EV-103 cohort L was similar to cohort H, but included perioperative treatment with enfortumab vedotin in cisplatin ineligible patients with muscle invasive bladder cancer:
Pathologic complete response rate in this trial was 34%, with a pathologic downstaging (< pT2) rate of 42%. Dr. Sridhar highlighted that there are many ongoing perioperative trials with enfortumab vedotin combinations:
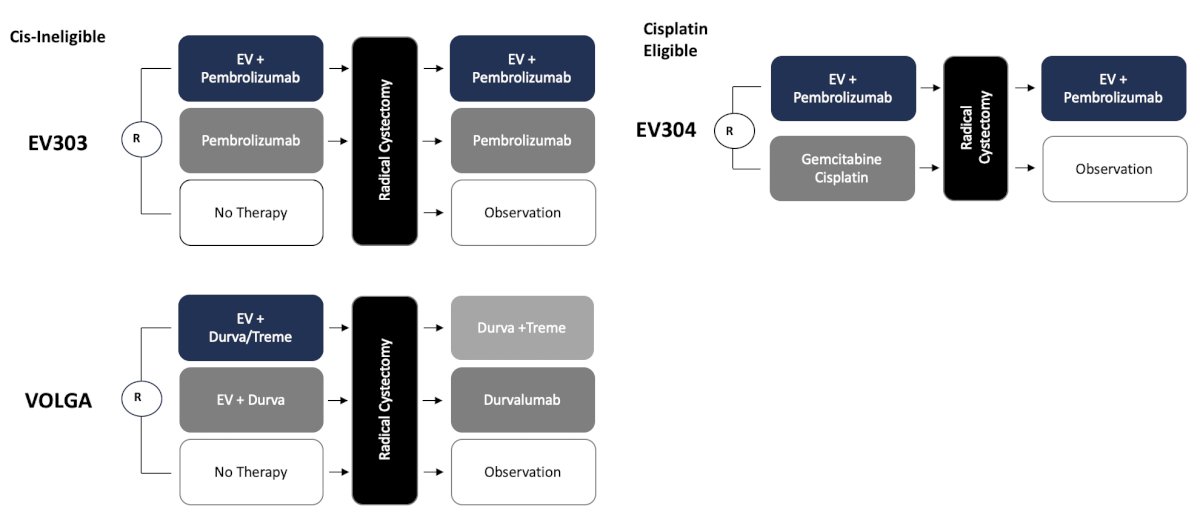
So, can we also start designing trials that allow for bladder sparing to be a consideration? Because of the success in the advanced disease setting, enfortumab vedotin is also being tested in the EV-104 trial via intravesical instillation in patients with BCG unresponsive CIS +/- papillary disease:
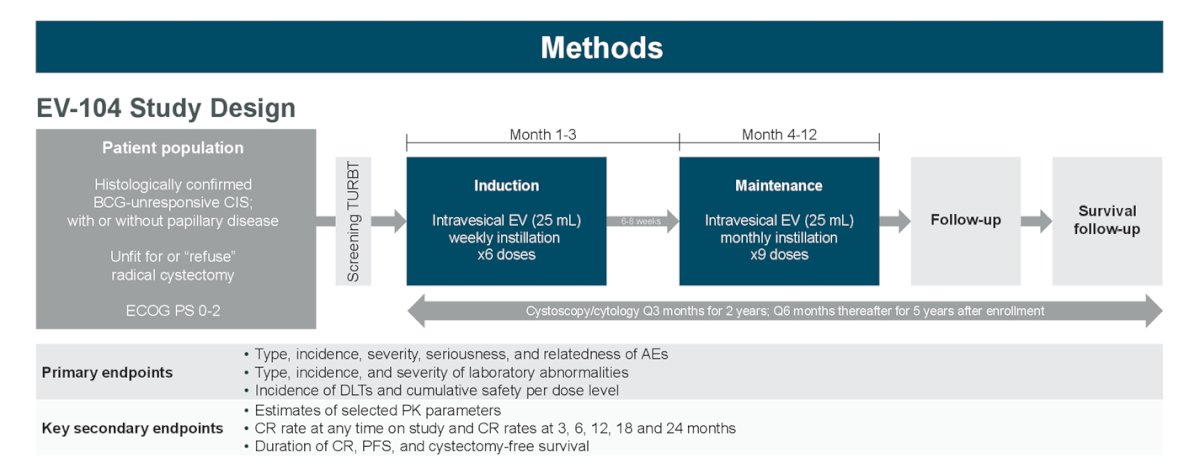
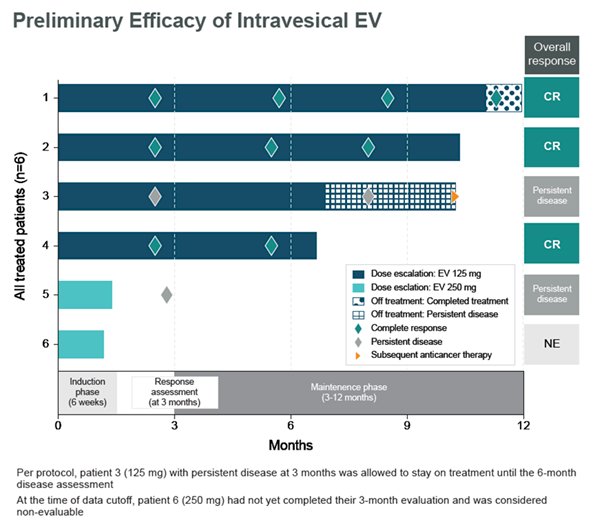
At ASCO 2023, data was presented on 6 patients receiving intravesical enfortumab vedotin across 2 dose levels: 4 patients at 125mg completed 6 induction cycles and proceeded to maintenance; 2 patients at 250mg had received at least 3 doses of intravesical enfortumab vedotin. Of 6 patients across doses, there were no Grade ≥3 treatment related adverse events, treatment-related symptomatic adverse events, treatment related adverse events leading to dose reduction, or discontinuation. Additionally, 4 of 6 patients (3 at 125mg and 1 at 250mg) experienced ≥1 treatment-related adverse event of Grade 1 or 2. The most common treatment related adverse events were fatigue (3 of 6) and micturition urgency (2 of 6). As of the data cutoff, there were no dose limiting toxicities observed for either 125 mg or 250mg. All blood PK analyses (antibody drug conjugate and unconjugated MMAE) for patients at 125mg were undetectable; no PK data was available for 250mg. Of 4 patients receiving 125mg of intravesical enfortumab vedotin, 3 achieved complete response and continue in response. The fourth patient discontinued treatment due to persistent disease but remains on study:
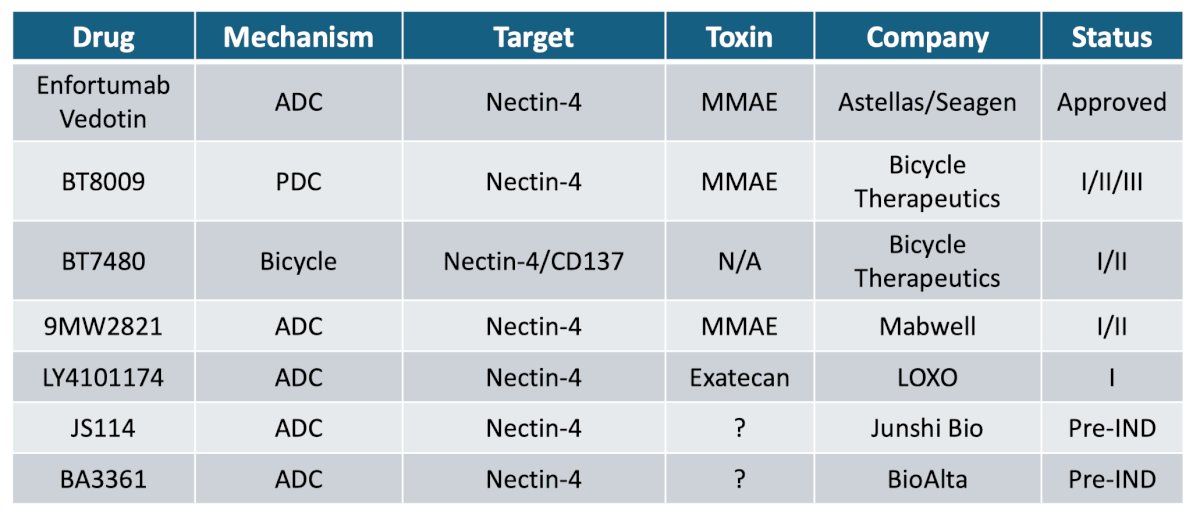
There are several nectin-4 agents in development, with different toxicity profiles:
Dr. Sridhar then discussed trop 2 and the antibody drug conjugate sacituzumab govitecan. Trop-2 is an epithelial cell surface antigen that is highly expressed in urothelial carcinoma. Sacituzumab govitecan is distinct from other antibody drug conjugates, with a high drug to antibody ratio and a bystander effect due to hydrolysable linker hydrolysis. There is significant activity across tumors and is approved for metastatic triple negative breast cancer. TROPHY-U-01 is a multi-cohort phase 2 trial, with cohort 1 (~100 patients) comprising patients with metastatic urothelial carcinoma who progressed after prior platinum-based and checkpoint inhibitor based therapies and subsequently treated with sacituzumab govitecan:
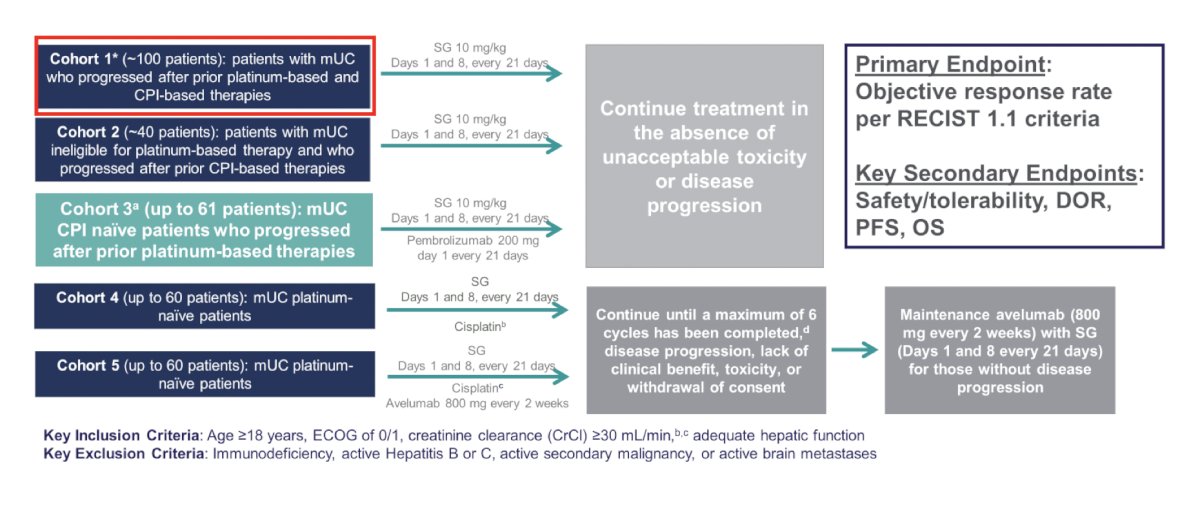
In cohort 1, sacituzumab govitecan had an objective response rate of 27% and at a median follow-up of 6.3 months, the median progression free survival was 5.4 months (95% CI 3.5-6.9) and the median overall survival was 10.5 months (95% CI 8.2-12.3). Based on this data, sacituzumab govitecan received accelerated approval. From a safety standpoint, 6% of patients discontinued therapy secondary to treatment related adverse events, 30% used G-CSF, and there was one treatment-related death:
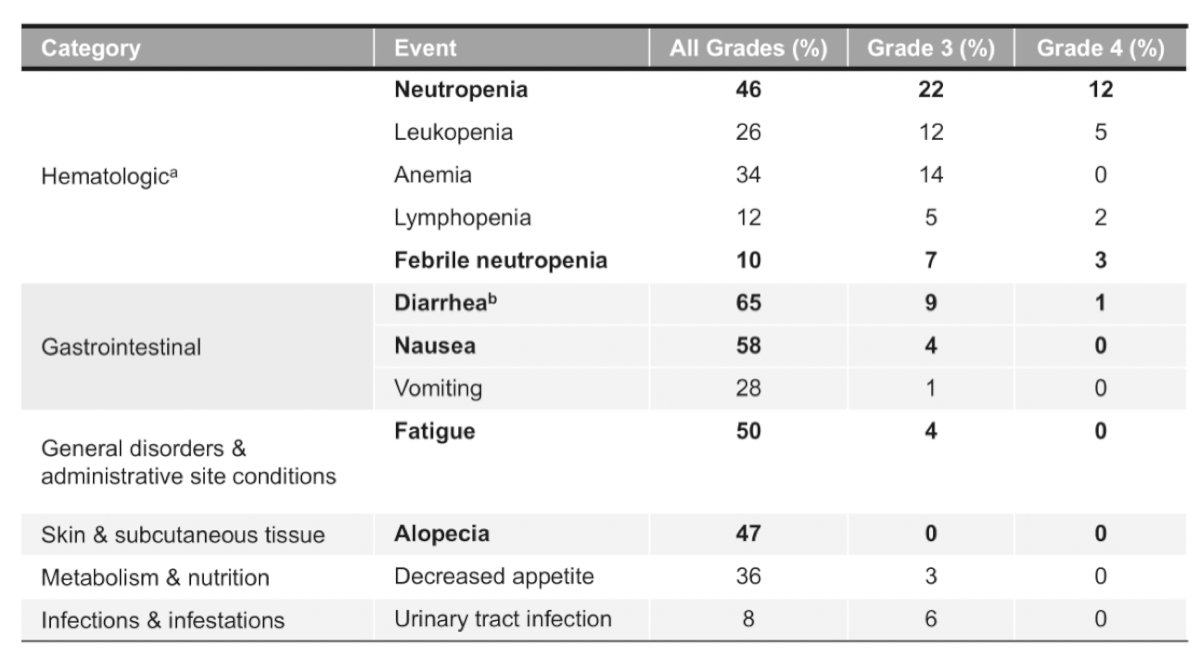
TROPHY-U-01 cohort 2 includes ~40 patients with metastatic urothelial carcinoma who progressed after checkpoint inhibitor therapy and were platinum ineligible at the start of the study. Among these patients, the objective response rate was 32%, median progression free survival was 5.6 months (95% CI 4.1-8.3) and median overall survival was 13.5 months (95% CI 7.6-15.6). In cohort 2, 68% of patients had grade >= 3 treatment related adverse events, most commonly neutropenia (34%), anemia (21%), leukopenia (18%), fatigue (18%), and diarrhea (16%).
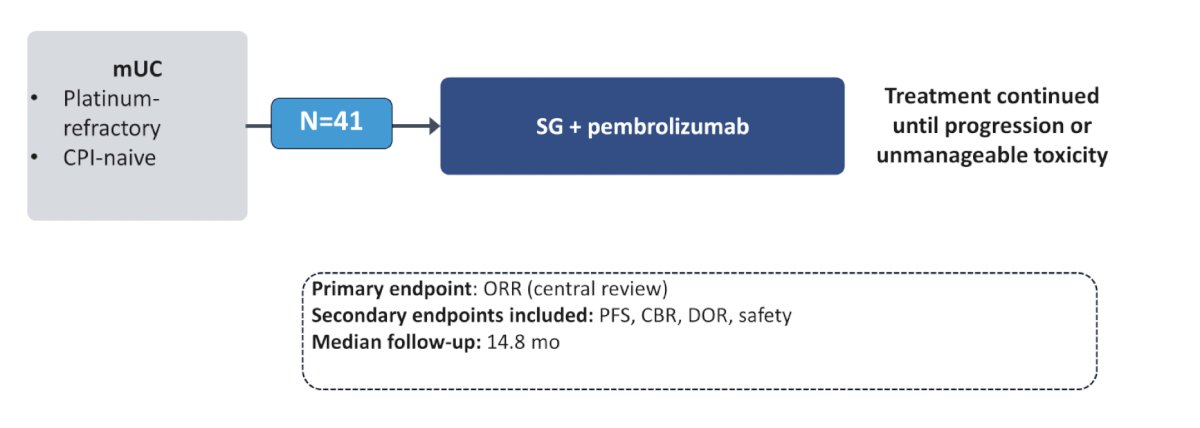
TROPHY-U-01 cohort 3 includes metastatic urothelial carcinoma patients that are platinum refractory and checkpoint inhibitor naïve that will receive sacituzumab govitecan + pembrolizumab:
In this cohort, objective response rate was 41%, with 20% complete response rate:
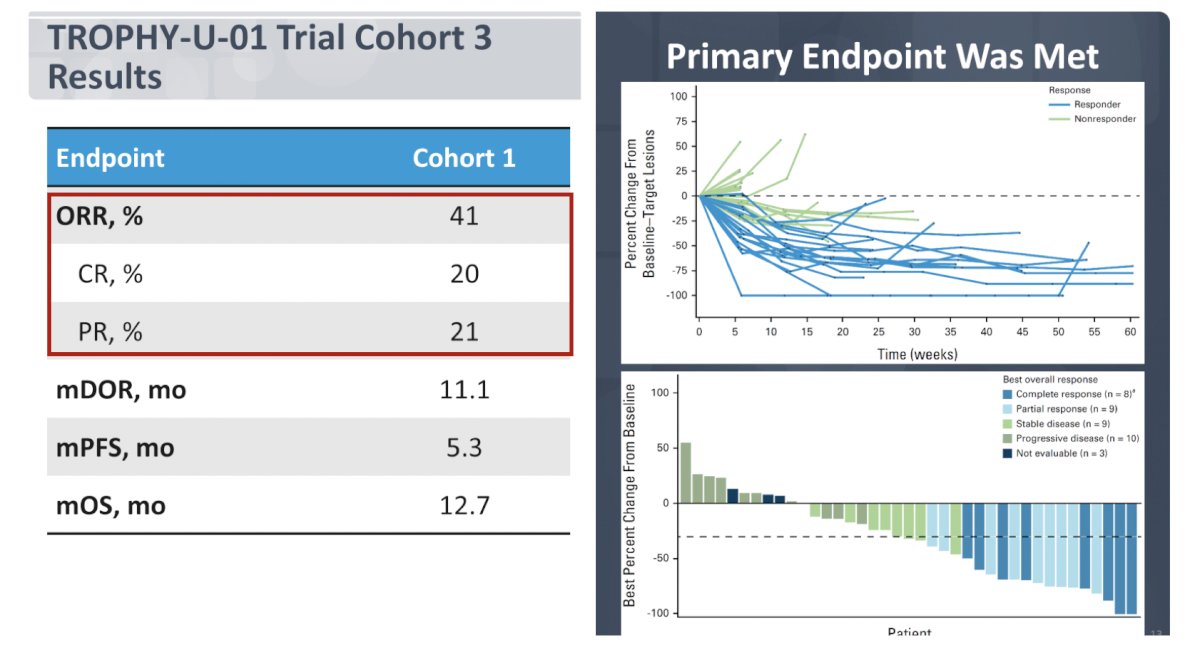
The most common treatment related adverse event of grade >= 3 (>= 5%) was neutropenia at 37%. Based on all of the aforementioned sacituzumab govitecan trials, there is now the phase 3 TROPICS-04 trial, which has a similar trial design as EV-301 and may potentially be used in sequence with enfortumab vedotin:
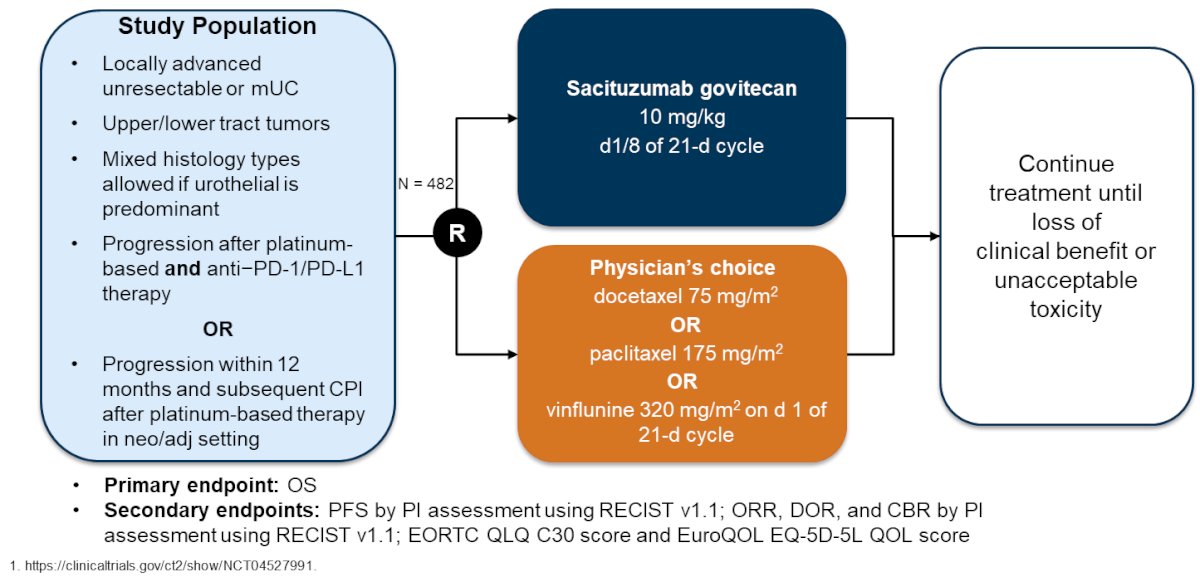
However, via press release on May 30, 2024, it was noted that TROPICS-04 did not meet the primary endpoint of overall survival in the intention to treat population, although there was a numerical improvement in overall survival.
Dr. Necchi is leading the SURE 01 trial assessing the utilization of neoadjuvant sacituzumab with the following trial design and primary endpoint results:
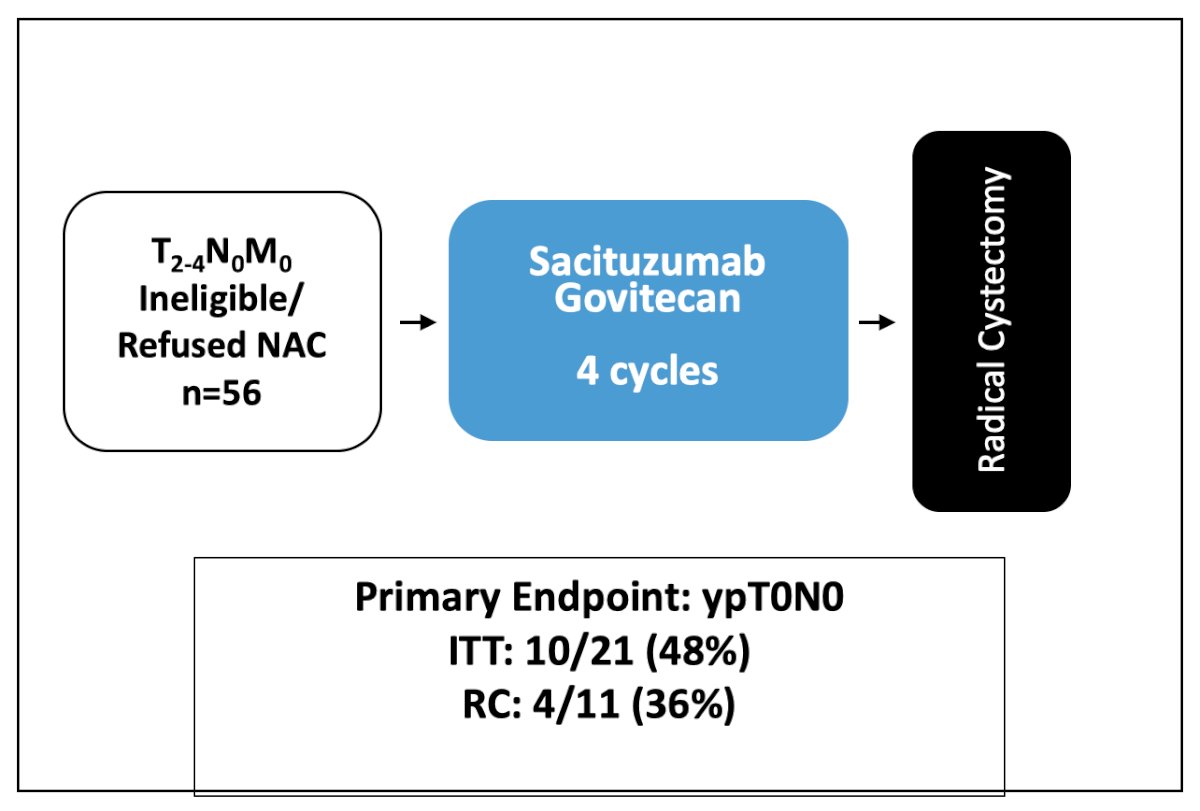
There were several key protocol changes noted, including a dose reduction of sacituzumab govitecan to 7.5 mg/kg from C1D1, the introduction of G-CSF use as primary prophylaxis since C1D9, and exclusion of patients who had >= 3 risk factors for febrile neutropenia as indicated by the ASCO guidelines. Interestingly, six patients had a clinical complete response (cystoscopy, MRI, and ctDNA) and subsequently declined radical cystectomy. Thus, Dr. Sridhar notes we need trials to account for this.
Another Trop 2 antibody drug conjugate is datopotamab deruxtecan (Dato DXd), which has three components:
- A humanized anti-TROP2 IgG1 monoclonal antibody attached to:
- A topoisomerase I inhibitor payload, an exatecan derivative, via
- A tetrapeptide-based cleavable linker
The phase 1 TROPION-PanTumor01 study included 33 patients with urothelial carcinoma and with the following trial design:
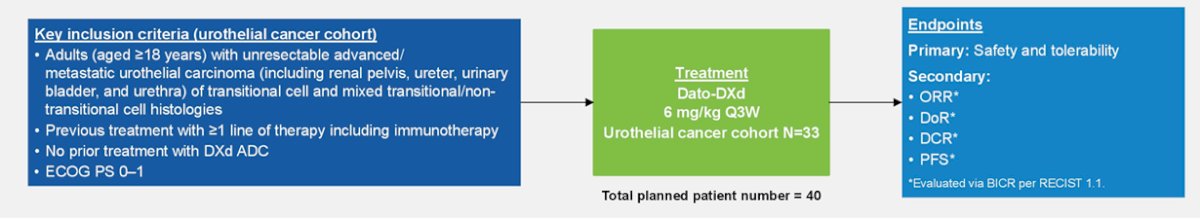
The objective response rate in this cohort was 19.2%, notably with 76% of metastatic urothelial carcinoma patients with >= 3 prior lines of therapy. The most common adverse events in this trial was stomatitis, nausea and fatigue.
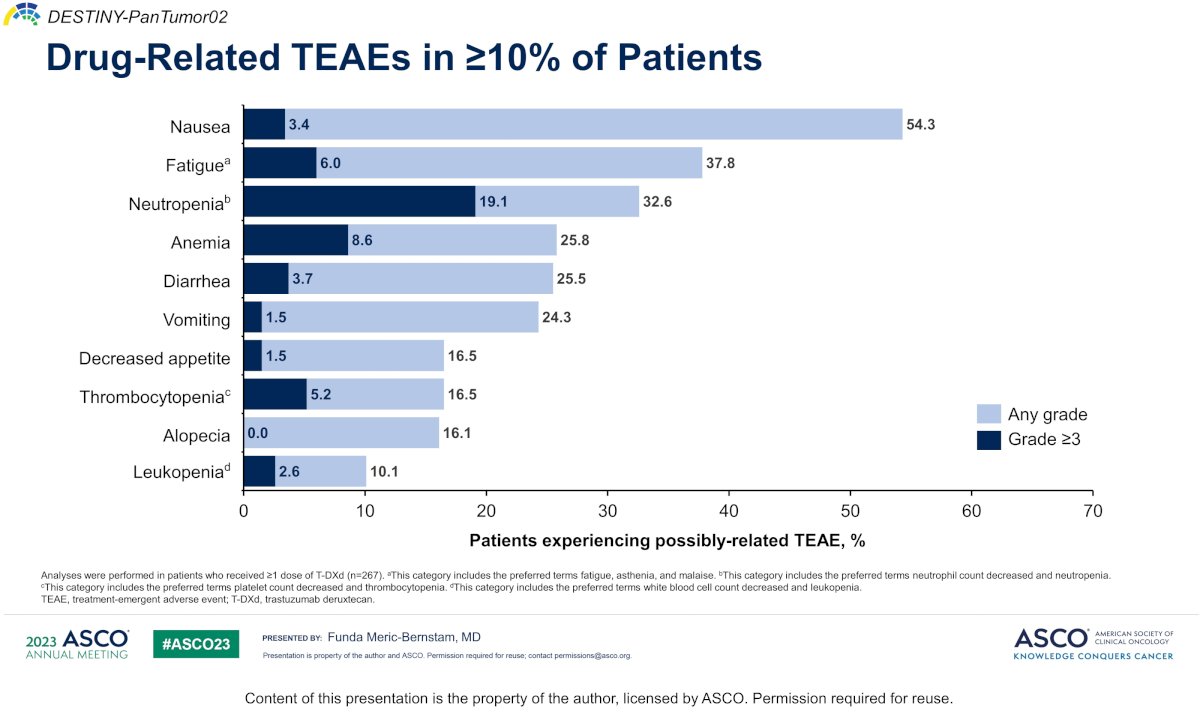
Dr. Sridhar then discussed trastuzumab deruxtecan (targeting HER2), which was assessed in the Destiny-PanTumor02 trial of HER2 expressing tumors, with a 39% objective response rate among urothelial carcinoma patients, but up to 56.3% for IHC3+ patients. Among drug-related treatment emergent adverse events in >=10% of patients, the most common were nausea, fatigue, and neutropenia:
In the DS8201-A-U105 study, trastuzumab deruxtecan was combined with nivolumab in HER2-expreessing advanced metastatic urothelial carcinoma:
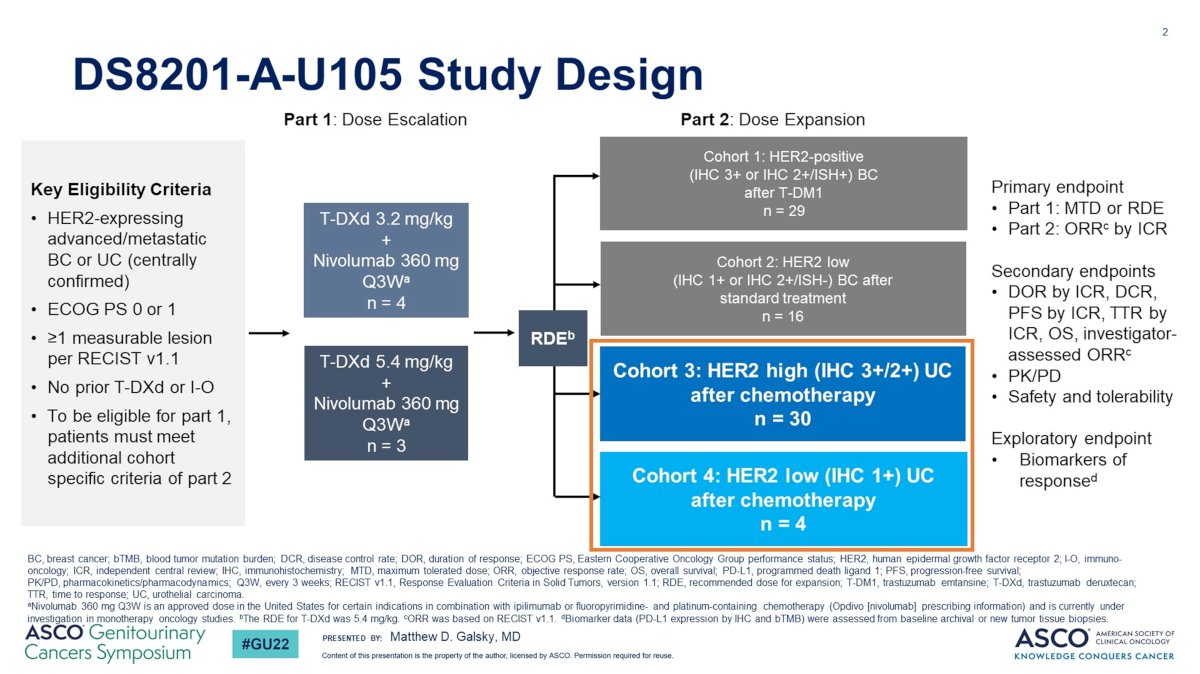
In this trial, among HER2 IHC 3+/2+, the confirmed objective response rate was 36.7% and median duration of response was 13.1 months (95% CI 4.1-not evaluable). Disitamab vedotin is an antibody drug conjugate directed at HER2, with the following mechanism of action:
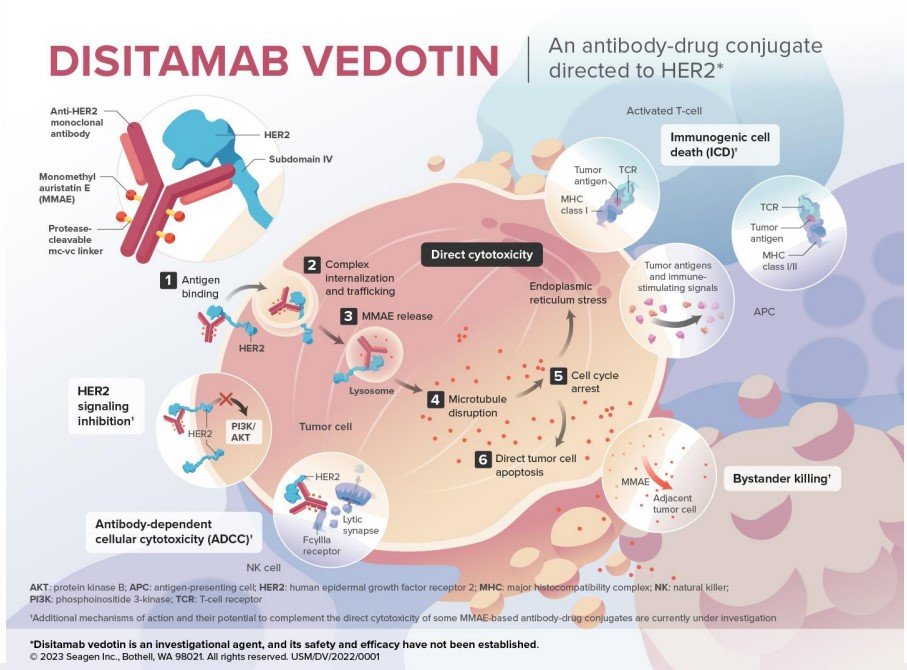
Early studies of disitamab vedotin suggest activity in both the HER2+/3+ (objective response rate 62.2%) and HER1+ (objective response rate 26.3%) cohorts: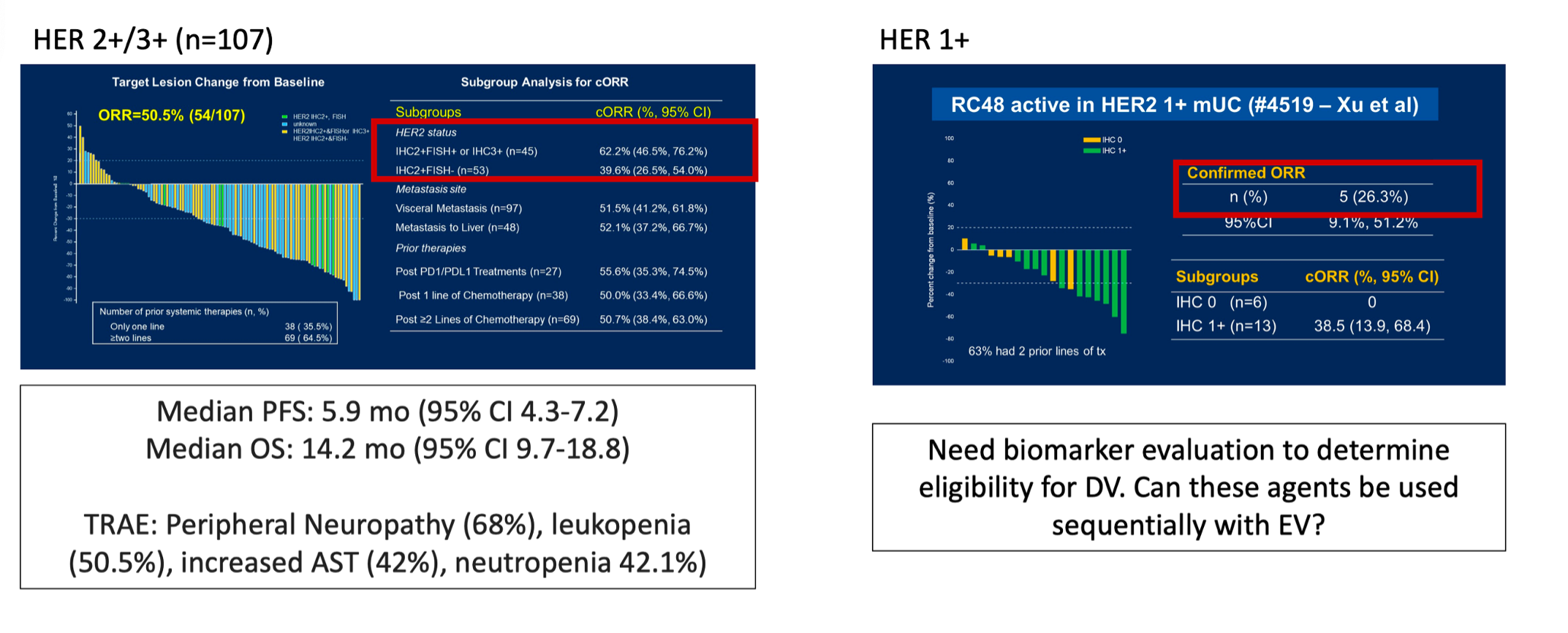
DV-001 is a phase 3 trial in progress assessing disitamab vedotin + pembrolizumab versus chemotherapy in first line locally advanced and metastatic urothelial carcinoma that expresses HER2: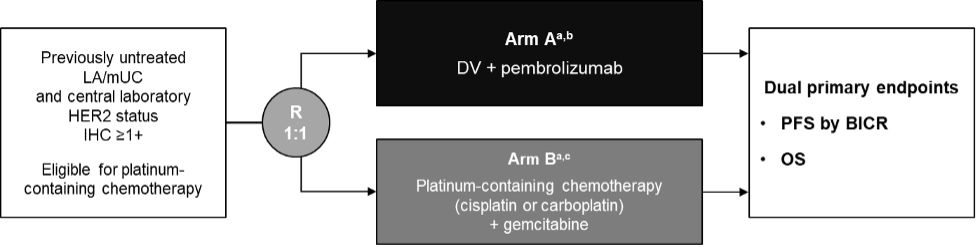
However, Dr. Sridhar questions whether based on the results of EV-302, is the current comparator still reasonable? These are the challenges of rapid development in the advanced disease space. Finally, Dr. Sridhar discussed the EGFR x HER3 bispecific antibody drug conjugate BL-B01D1: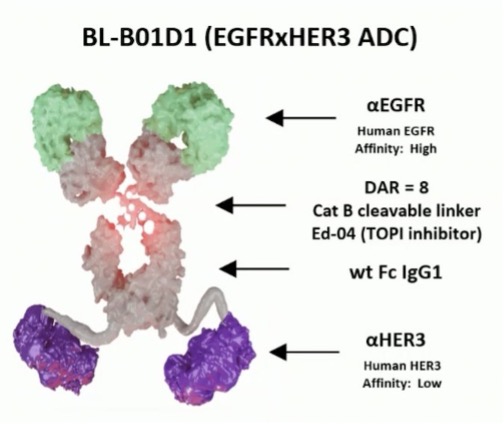
Preliminary data was presented at ESMO 2024, noting that evidence of antitumor activity was present, with an objective response rate of 40.7%. Moreover, 9/12 (75%) with only one prior line of therapy responded, and treatment was tolerable with hematologic toxicity being the most common adverse event. There was no interstitial lung disease or skin toxicity reported. The following table highlights objective response rates for antibody drug conjugates, noting that the MMAE payloads appear to have higher objective response rates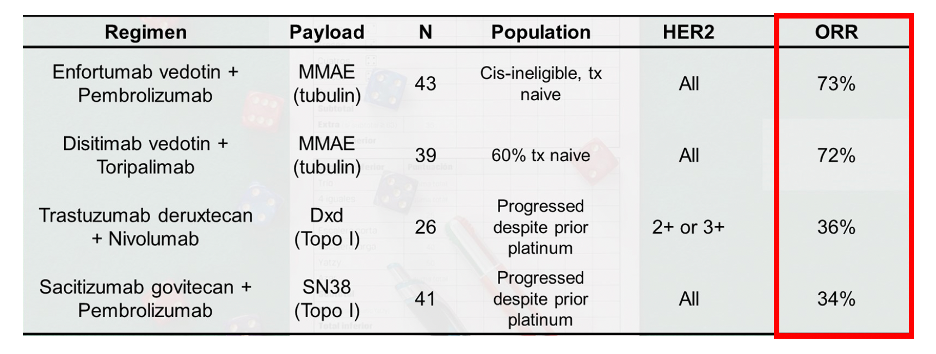
Dr. Sridhar concluded her presentation discussing antibody drug conjugate toxicity and management with the following take-home points:
- There have been significant practice changing advances in the field
- Antibody drug conjugates are changing how we treat metastatic disease, and being evaluated in the muscle invasive and NMIBC settings
- We urgently need biomarker testing to determine if patients are eligible for some of these treatments (ie. HER2 targeted)
- We need to understand how best to sequence treatments to maximize response
- It is a very exciting time in the field, offering new hope for our patients
Presented by: Srikala Sridhar, Princess Margaret Cancer Centre, Toronto, Ontario, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024
References:
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10)875-888.


