(UroToday.com) The 2024 IBCN annual meeting included a session on molecular subtyping in 2024, featuring a presentation by Dr. Jon Griffin discussing the trial in progress, GUSTO. The key question to be answered in GUSTO is: Will gene expression subtype guided neoadjuvant chemotherapy and/or immunotherapy better than unselected neoadjuvant treatment in patients with bladder cancer eligible for radical cystectomy? Additionally, there are several other outstanding questions:
- Is gene expression subtyping feasible within clinical timeframes?
- Is recruitment feasible for this type of trial?
- Does the gene expression subtype distribution match that in other populations?
- How do the different gene expression classifications compare?
- Is there a difference in outcome by gene expression subtype?
The trial design for GUSTO is as follows, emphasizing the treatment pathway allocation based on subtype selection care: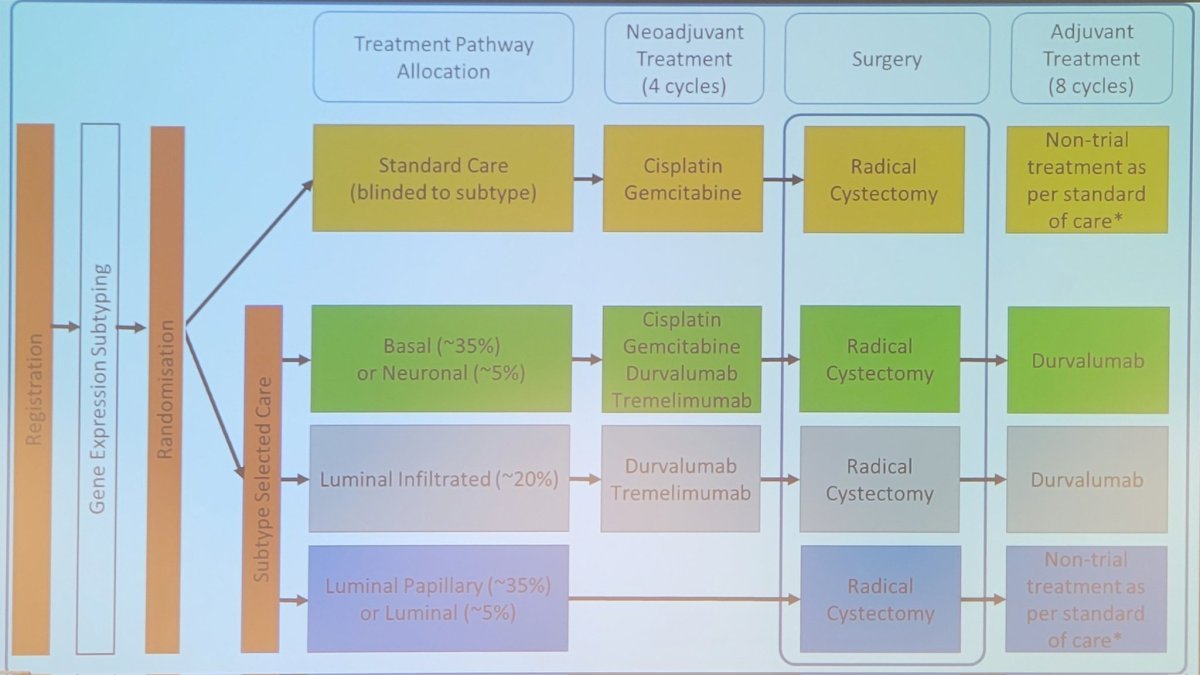
This trial will recruit 320 patients over 3 years at 20 sites in the United Kingdom in 3 stages:
- Stage 1: feasibility of recruitment and subtype turnaround time (0-6 months; n = 30). This stage has been completed
- Stage 2: confirm subtype distributions and pathologic complete response rates +/- sample size recalculation (7-24 months; n = 202). This stage is ongoing
- Stage 3: pathologic complete response rate by subtype (24-36 months; n = 320)
Secondary endpoints include 12-month disease-free survival, overall survival, metastasis-free survival, event-free survival, quality of life, and tolerability + toxicity. The workflow for an individual patient in GUSTO is as follows: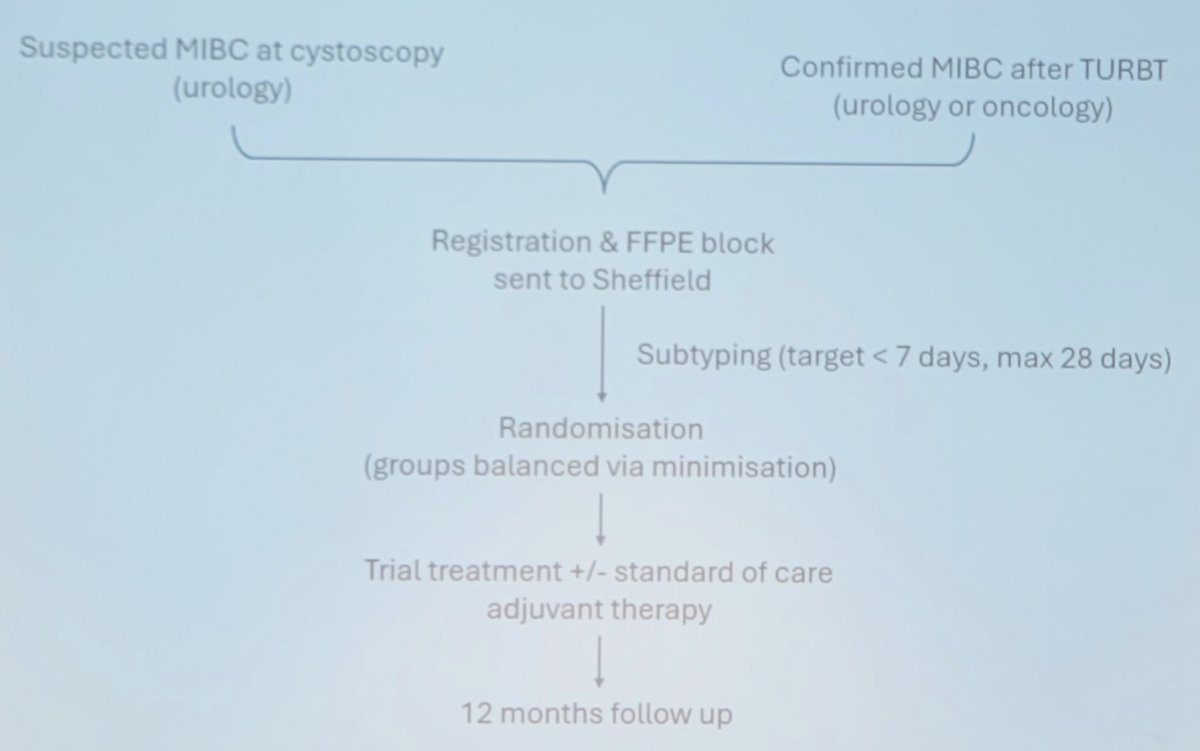
Of note, there will be translational lab studies evaluated, at the following time points:
- Pre neoadjuvant chemotherapy
- Post neoadjuvant chemotherapy
- 3 months post radical cystectomy
- 12 months post radical cystectomy
Presented by: Jon Griffin, NIHR Clinical Lecturer, The University of Sheffield, Sheffield, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024


