(UroToday.com) The 2024 IBCN annual meeting included a session on molecular subtyping in the 2024, featuring a presentation by Dr. Saum Ghodoussipour discussing the LUMBER-NAC trial in progress. A standard treatment of muscle-invasive bladder cancer is neoadjuvant chemotherapy followed by radical cystectomy with pelvic lymph node dissection.
Based on contemporary series and meta-analyses, there is an overall survival benefit of neoadjuvant chemotherapy of 5-10%, however, response rates are variable among patients. Molecular subtyping may better clarify which patients with muscle-invasive bladder cancer respond best to neoadjuvant chemotherapy.
Lotan and colleagues previously assessed 601 patients with muscle-invasive bladder cancer, of whom 247 had been treated with neoadjuvant chemotherapy and radical cystectomy, and 354 underwent radical cystectomy without neoadjuvant chemotherapy.1 With neoadjuvant chemotherapy, the overall net benefit to overall survival and cancer-specific survival at 3 years was 7% and 5%, respectively. After controlling for clinicopathological variables, non-luminal tumors had greatest benefit from neoadjuvant chemotherapy, with 10% greater overall survival at 3 years (71% vs 61%), while luminal tumors had minimal benefit (63% vs 65%) for neoadjuvant chemotherapy vs non-neoadjuvant chemotherapy: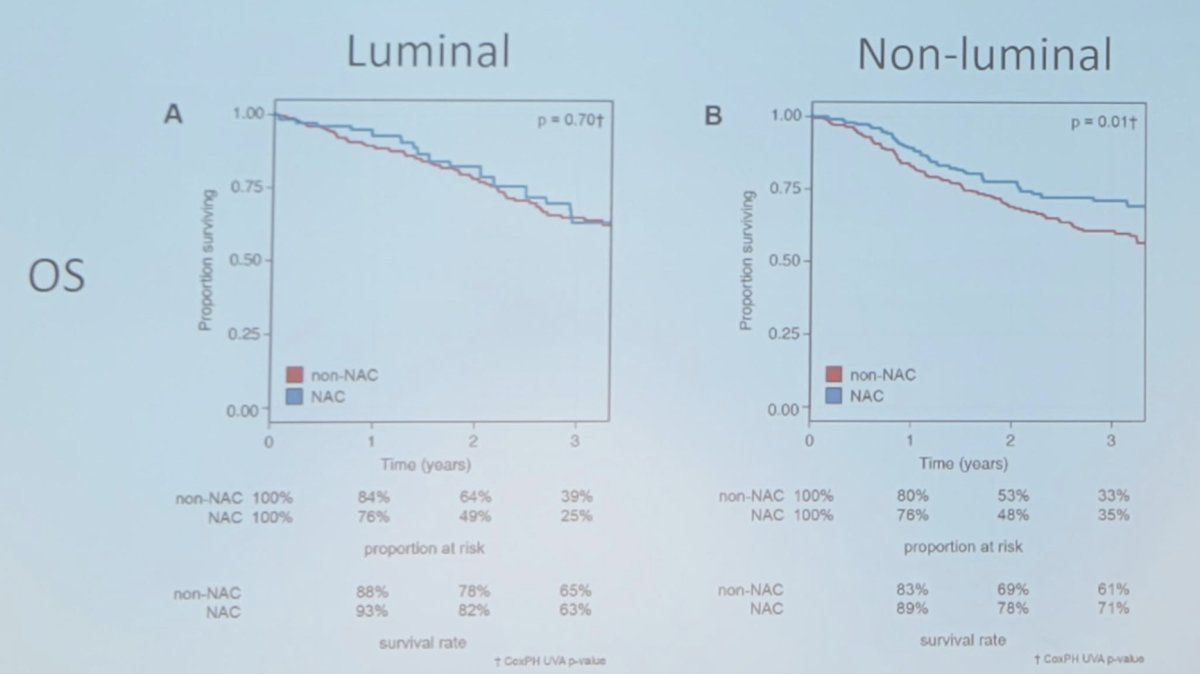
Dr. Ghodoussipour questions whether we can safely and effectively de-escalate therapy in appropriate candidates. This would potentially spare patients treatment-related morbidity, maximize quality of life, limit the time to curative treatment, and improve oncological outcomes. The study schema for the LUMBAR-NAC trial is as follows, highlighting the randomization of luminal subtype muscle-invasive bladder cancer to undergo versus not undergo neoadjuvant chemotherapy prior to radical cystectomy: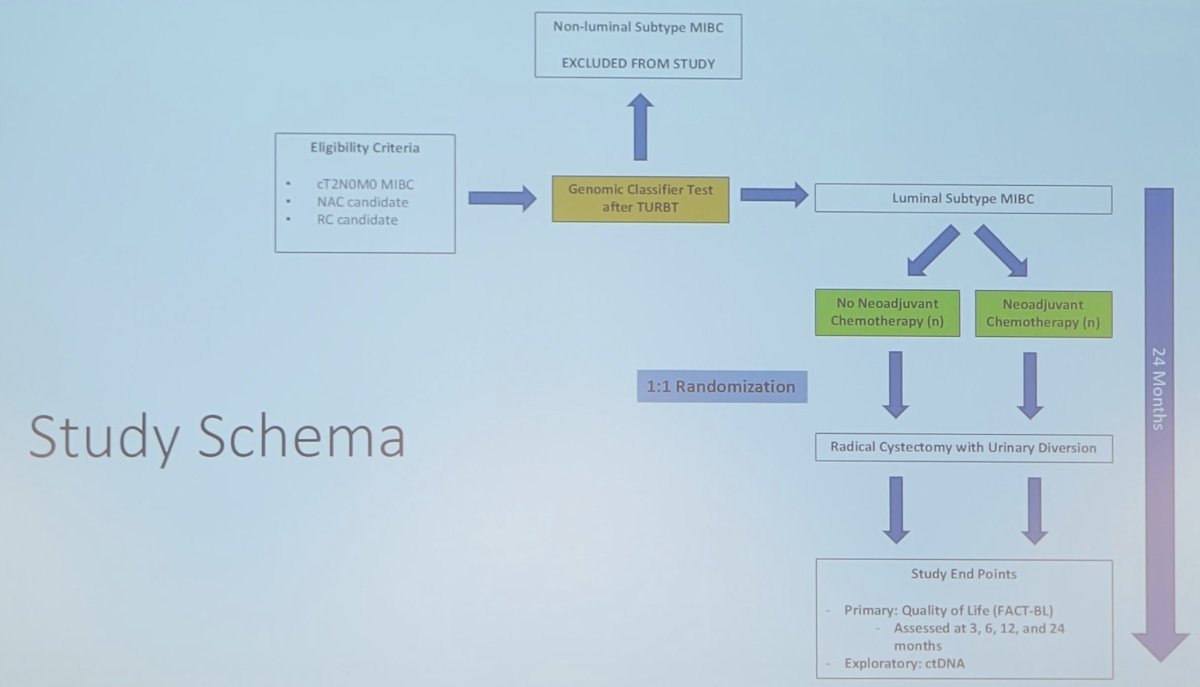
Dr. Ghodoussipour notes that Veracyte’s minimal residual disease approach, powered by C2i, provides a highly sensitive, data-rich platform for diagnostics and discovery. There are several notable advantages:
- Faster speed to answer: bespoke panels can require 6+ weeks to begin monitoring, which is too long for some treatment decisions
- Improved sensitivity: with targeted approaches, you can only monitor for mutations on the limited panel
- Lower sample requirements: targeted approaches look for a needle in the haystack, requiring larger volumes to capture rare events
- More data for discovery: data from small panels could limit the potential future research use
Dr. Ghodoussipour concluded his presentation discussing the LUMBER-NAC trial by highlighting the potential impact:
- Provides prospective validation of a genomic subtype classifier
- Provides bench to bedside in a meaningful way for patients
- Has scientific potential, providing sample collection, further correlates, and validation of a novel minimal residual disease assay
Presented by: Saum Ghodoussipour, MD, Urologist, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024
References:- Lotan Y, de Jong JJ, Liu VYT, et al. Patients with muscle-invasive bladder cancer with nonluminal subtype derive the greatest benefit from platinum-based neoadjuvant chemotherapy. J Urol. 2022 Mar;207(3):541-550.


