(UroToday.com) The 2024 IBCN annual meeting included a session on molecular subtyping in the 2024, featuring a presentation by Dr. Yves Allory discussing the VESPER trial. The GETUG-AFU V05 VESPER trial is a randomized phase III trial of 500 patients with cT2-4aN0M0 urothelial carcinoma of the bladder receiving neoadjuvant chemotherapy or pT3-4 or pTanyN+Many receiving adjuvant chemotherapy.
Included patients were randomized to either six cycles of dose-dense MVAC once every two weeks or four cycles of gemcitabine and cisplatin once every three weeks before surgery (neoadjuvant group) or after surgery (adjuvant group). Results of the primary endpoint outcome of three-year progression-free survival were published in 2022.1 Of the 493 patients in the intent-to-treat population, 88.6% received chemotherapy in the neoadjuvant setting, with the remaining 11.4% receiving adjuvant chemotherapy. Median patient age was 63.0 years. Three-year progression-free survival in patients receiving neoadjuvant chemotherapy was significantly higher in the dose-dense MVAC arm (66% versus 56%; HR 0.70, 95% CI 0.51 to 0.96, p = 0.025):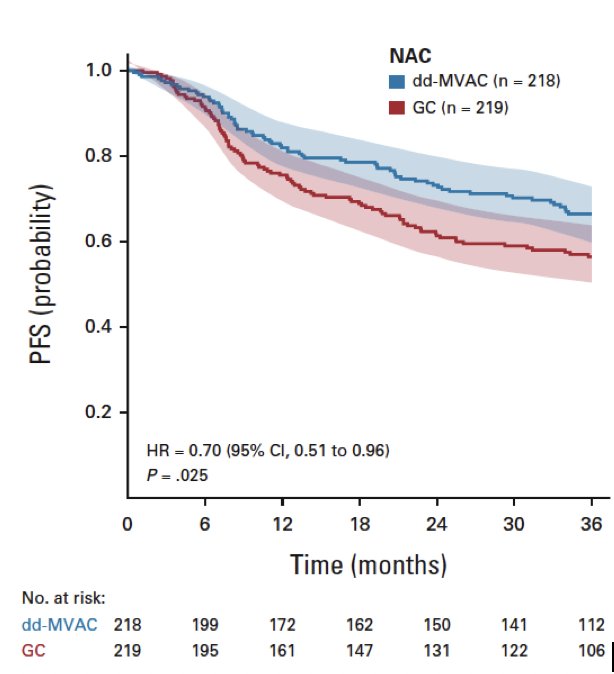
Overall survival among patients receiving neoadjuvant chemotherapy was in favor of dose-dense MVAC (HR 0.71, 95% CI 0.52 to 0.97):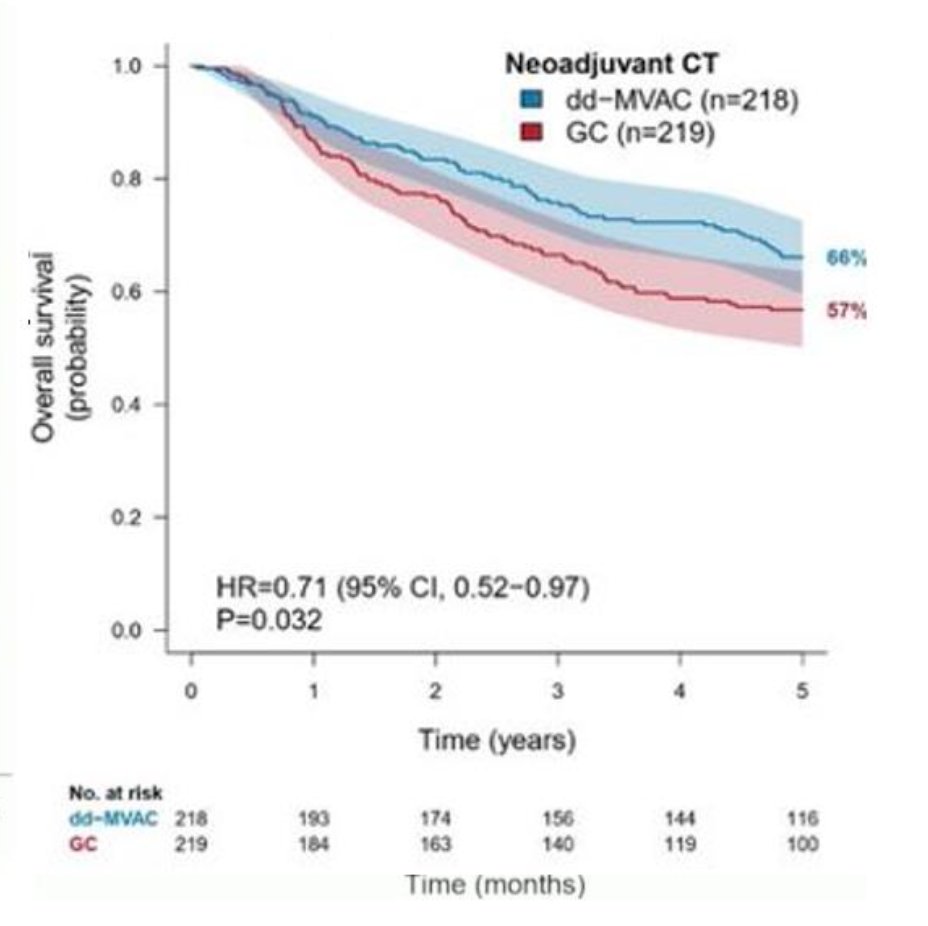
The extended follow-up of the VESPER trial was published earlier in 2024 [2], noting that in the neoadjuvant subgroup, overall survival at 5 years was improved in the dose dense MVAC group versus the gemcitabine + cisplatin group (66%, 95% CI 60-73 vs 57%, 95% CI 50-64; HR 0.71, 95% CI 0.52-0.97), as was time to death due to bladder cancer (5-year cumulative incidence: 24%, 95% CI 18-30 vs 38%, 95% CI 32-45; HR 0.55, 95% CI 32-45 0.39-0.78). The study design schema for the molecular subgroup analysis is as follows: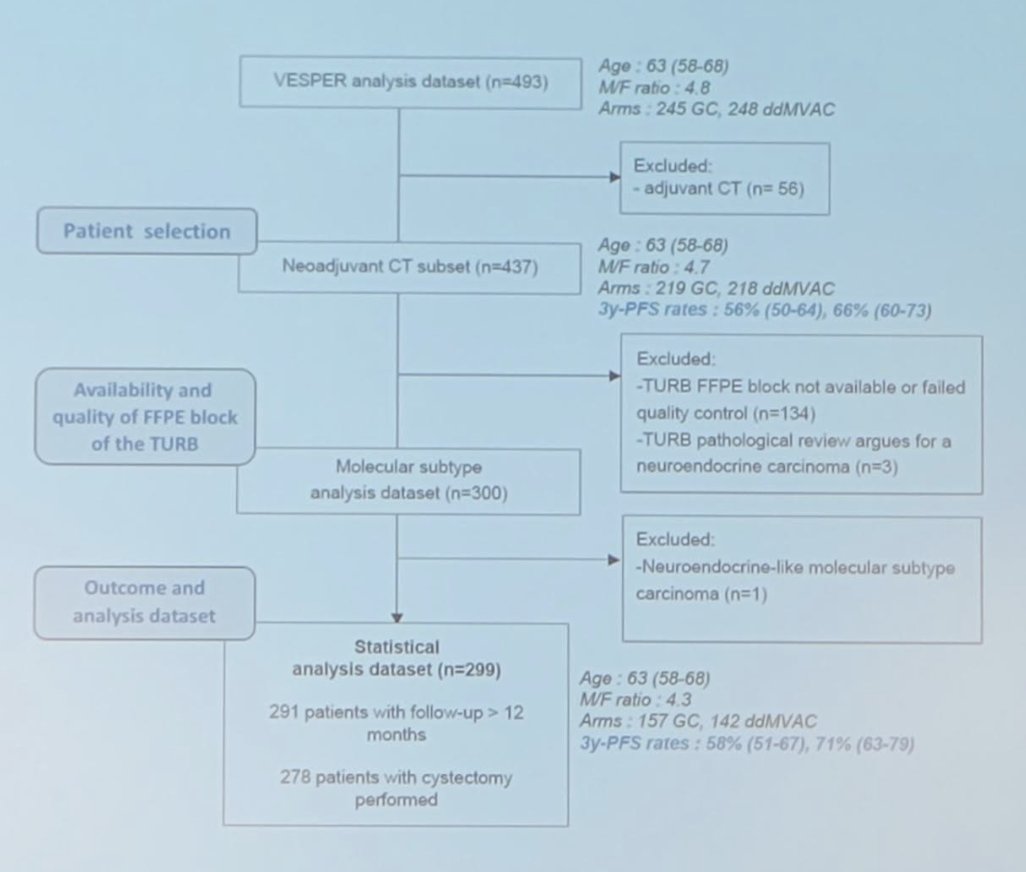
The pathological complete response rate stratified by subgroup is as follows: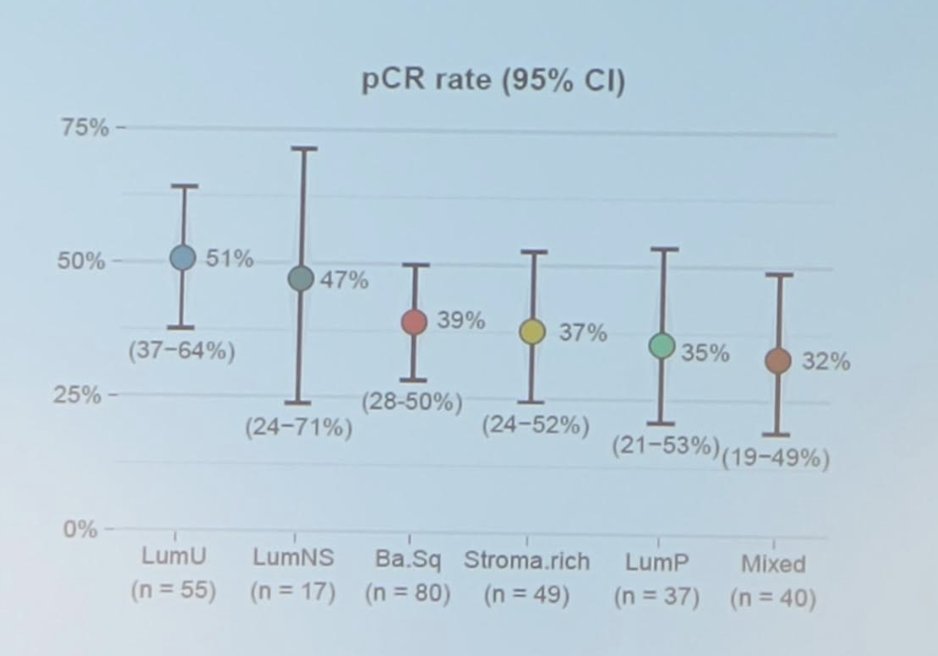
This study found that the BaSq subtype (either pure or mixed) was associated with decreased progression free survival and overall survival compared to the other subtypes in the VESPER trial, regardless of the chemotherapy arm: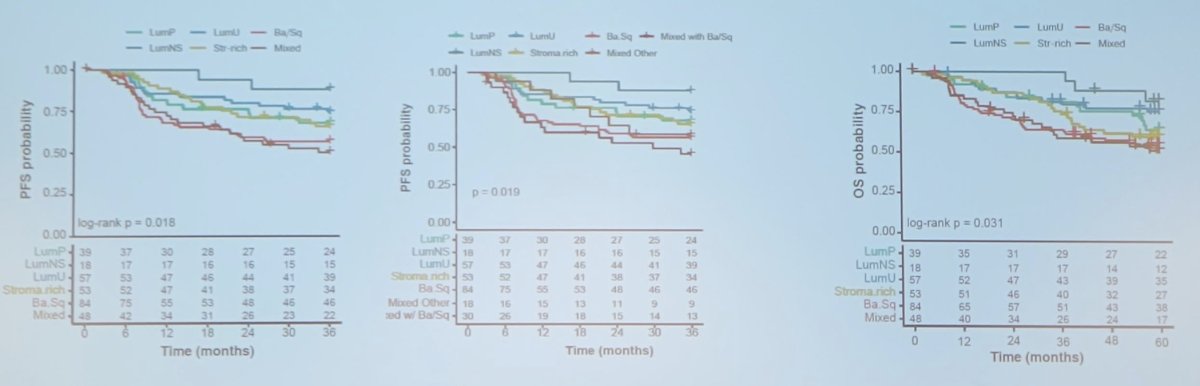
Additionally, in a multivariable Cox model, the BaSq subgroup was associated with progression free survival (HR 2.0, 95% CI 1.36-3.0).
Dr. Allory concluded his presentation discussing the VESPER trial molecular subtyping by highlighting several remaining questions:
- What are the biological bases for the mixed tumors?
- How do we handle clinical heterogeneity detection?
- How do we improve BaSq (pure or mixed) tumor prognosis?
Presented by: Yves Allory, Institut Curie, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024
References:
- Pfister C, Gravis G, Flechon A, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: Results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022 Jun 20;40(18):2013-2022.
- Pfister C, Gravis G, Flechon A, et al. Perioperative dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer (VESPER): Survival endpoints at 5 years in an open-label, randomized, phase 3 trial. Lancet Oncol. 2024 Feb;25(2):255-264.


