(UroToday.com) The 2024 IBCN annual meeting included a session on treatment response correlates, featuring a presentation by Dr. Aymeric Zadoroznyj discussing the systematic evaluation of differentially expressed genes associated with response to neoadjuvant chemotherapy in muscle invasive bladder cancer. Neoadjuvant cisplatin-based combination chemotherapy is standard of care for patients with muscle-invasive bladder cancer. Around one-third of patients achieve a pathologic complete response (ypT0N0) and have excellent survival after radical cystectomy. Predicting pathologic complete response based at TURBT would enable selective administration of neoadjuvant chemotherapy, sparing patients from side effects and exposure to ineffective therapy. At IBCN 2024, Dr. Zadoroznyj and colleagues aimed to identify genes that robustly predict pathologic complete response in published transcriptomes and integrate data from several studies to prioritize genes for experimental validation.
The goal of this study was to analyze the largest transcriptomic dataset of bladder cancer to highlight potential subtype-specific biomarkers in neoadjuvant chemotherapy. The datasets included had transcriptomic TURBT profiles from neoadjuvant chemotherapy-treated patients (n>50, >1 cycle MVAC or gemcitabine + cisplatin). The pipeline for evaluation of neoadjuvant chemotherapy biomarkers is as follows:
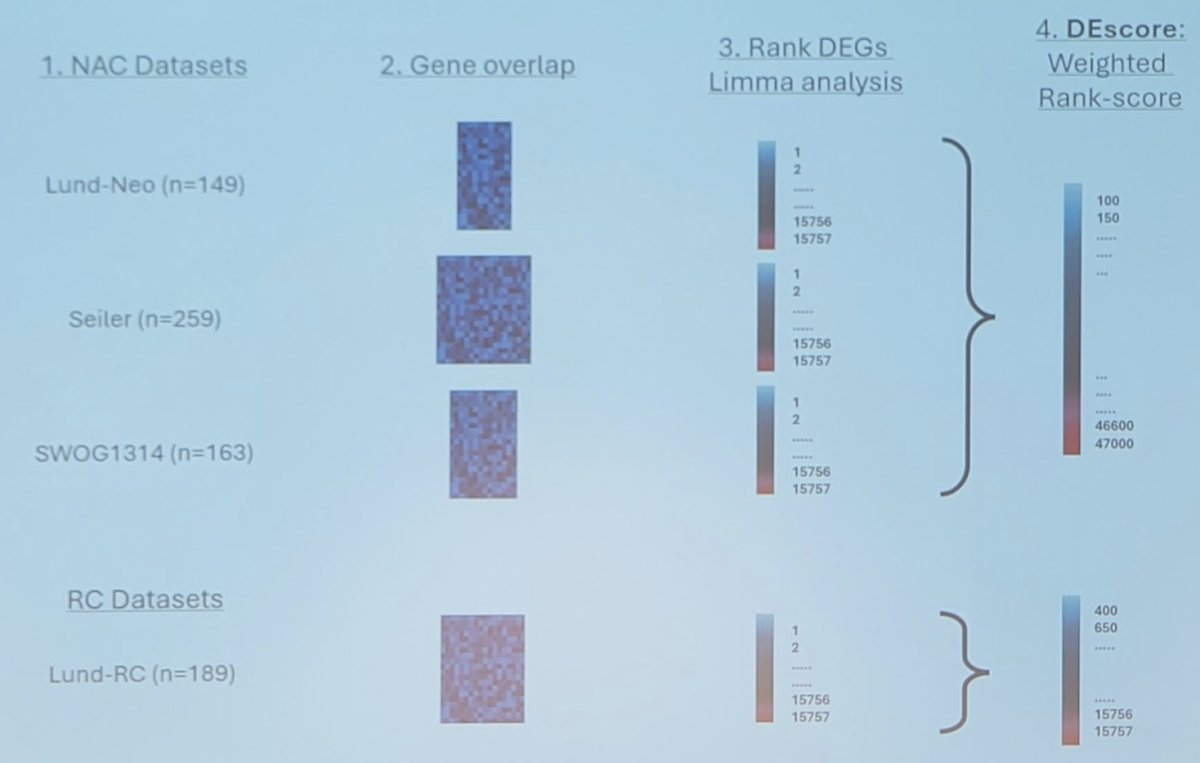
Microarray data were processed using updated annotations and probe-set definitions from the ‘brainarray’ package and differential expression analysis was performed using the ‘limma’ R package. Genes were assigned a differential expression rank in each cohort, and ranks were merged across cohorts weighted by sample size, number of significant genes, or in an unweighted manner. Merged rank-lists were filtered against results in a radical cystectomy-only dataset to flag genes associated with pT0N0 in the absence of neoadjuvant chemotherapy.
Three neoadjuvant chemotherapy cohorts were included: Seiler et al. (2017),1 n = 262; Sjödahl et al. [2] (2022), n = 125; and Lerner et al. [3] (2024), n = 168, and one radical cystectomy-only cohort, Sjödahl et al.4 (2018), n = 186. Differential expression analysis by pathologic complete response status was performed on 15,757 genes present in all cohorts. Top neoadjuvant chemotherapy-specific response associated genes using ‘limma’, weighted by significance, were KBTBD4, C9orf64, TK1, SMCO4, and NR1H3. Top resistance-associated genes were SDCCAG8, FGGY, CRCP, IFT57, and INO80D. GO term analysis of the Ba/Sq subtype demonstrated cytotoxic T-cells, NK cells, and immune response, whereas for the Uro subtype demonstrated cell proliferation and cell cycle.
Future perspectives include:
- Resampling tests
- Validating potential subtype-specific biomarkers by immunohistochemistry
- Functional analysis of biomarkers
- Maintaining rank tables to allow incorporation of future cohorts
Dr. Zadoroznyj concluded his presentation discussing the systematic evaluation of differentially expressed genes associated with response to neoadjuvant chemotherapy in muscle invasive bladder cancer with the following take-home points:
- The combination of multiple cohorts improves the robustness of differential expression analysis
- Very different results are obtained for molecular subtypes
- DEscore is a biomarker prioritization pipeline that will improve with new data
Presented by: Aymeric Zadoroznyj, MD, Lund University, Lund, Sweden
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024
References:- Seiler R, Al Deen Ashab H, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017 Oct;72(4):544-554.
- Sjodahl G, Abrahamsson J, Holmstein K, et al. Difference responses to neoadjuvant chemotherapy in urothelial carcinoma molecular subtypes. Eur Urol. 2022 May;81(5)523-532
- Lerner SP, McConkey DJ, Tangen CM, et al. Association of molecular subtypes with pathologic response, PFS, and OS in a phase II study of COXEN with neoadjuvant chemotherapy for muscle-invasive bladder cancer. Clin Cancer Res 2024 Jan 17;30(2):444-449.
- Sjodahl G, Eriksson P, Lovgren K, et al. Discordant molecular subtype classification in the basal-squamous subtype of bladder tumors and matched lymph-node metastases Mod Pathol
. 2018 Dec;31(12):1869-1881


