(UroToday.com) The 2024 IBCN annual meeting included a session on the current status and future directions of antibody-drug conjugates, featuring a presentation by Dr. Markus Eckstein discussing mechanisms of resistance to antibody-drug conjugates.
Dr. Eckstein started his presentation by noting that antibody drug conjugates independently target protein function, but are dependent on membranous target protein expression. There are several potential mechanisms of resistance, which may include:
- Lack of and restriction of target proteins
- Conventional – akin to chemotherapy resistance
- Remodeling of the microenvironment
Lack of and restriction of target proteins is one mechanism of action for sacituzumab govitecan resistance in triple-negative breast cancer: MGH18 TROP2 high is associated with partial response, whereas MGH20 TROP2 negative is associated with primary progressive disease. The following timeline highlights an anecdotal case from Dr. Eckstein’s molecular tumor board in the pre-enfortumab vedotin era: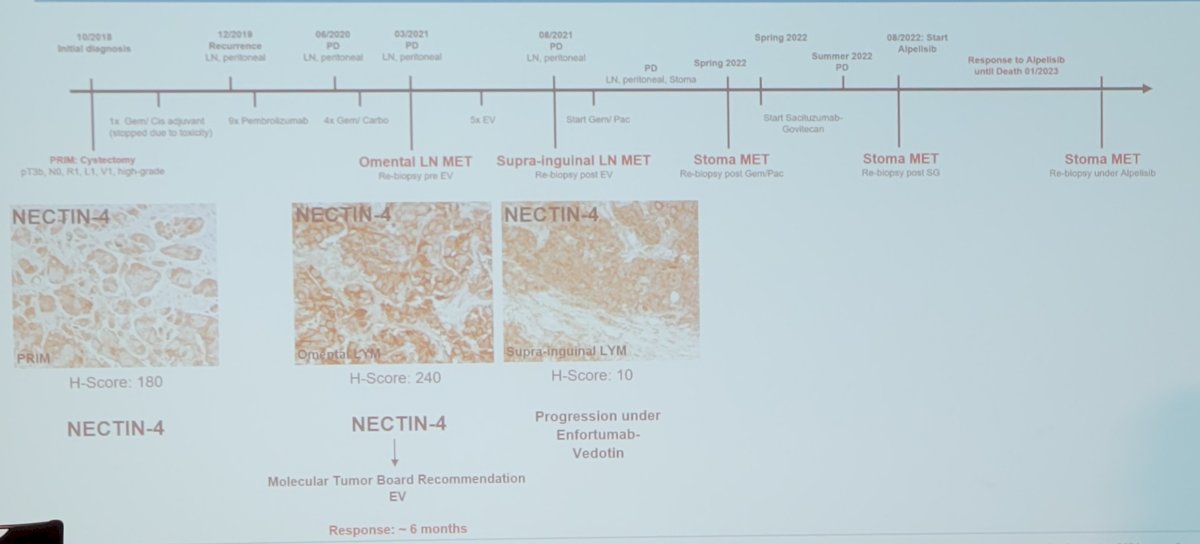
Lack of and restriction of target proteins (ie. Nectin-4) has also been assessed in clinical trials, notably the EV-101 trial.1 In EV-101, there was strong Nectin-4 IHC expression in >95% of patients with urothelial carcinoma, with a median H-score of 290, and 95% of tumors having an H-score >150:
Earlier in 2024, Dr. Eckstein’s group aimed to characterize associations of TACSTD2/TROP2 and NECTIN-4/NECTIN-4 protein and gene expression with morphomolecular and clinicopathological characteristics of advanced urothelial carcinoma.2 TROP2/TACSTD2 and NECTIN-4/NECTIN-4 were highly expressed at the protein and transcript level in advanced urothelial carcinoma, and their expression status did not correlate with patient survival. NECTIN-4/NECTIN-4 expression was higher in luminal tumors and reduced in squamous advanced urothelial carcinoma. Moreover, NECTIN-4 was negative in 10.6% of samples, 18.4% of samples had low expression (H-score <15), and the TROP2 negativity rate was 6.5%: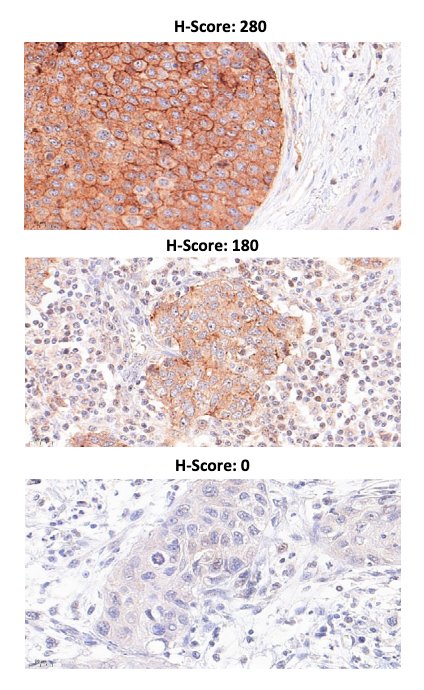
Dr. Eckstein then discussed the EV-302 trial,3 specifically the relationship with Nectin-4. Drs. Klumper and Eckstein previously showed that membranous Nectin-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance.4 In this analysis, they showed that the absence or weak membranous Nectin-4 expression (H-score 0-99) is associated with shorter progression-free survival on enfortumab vedotin: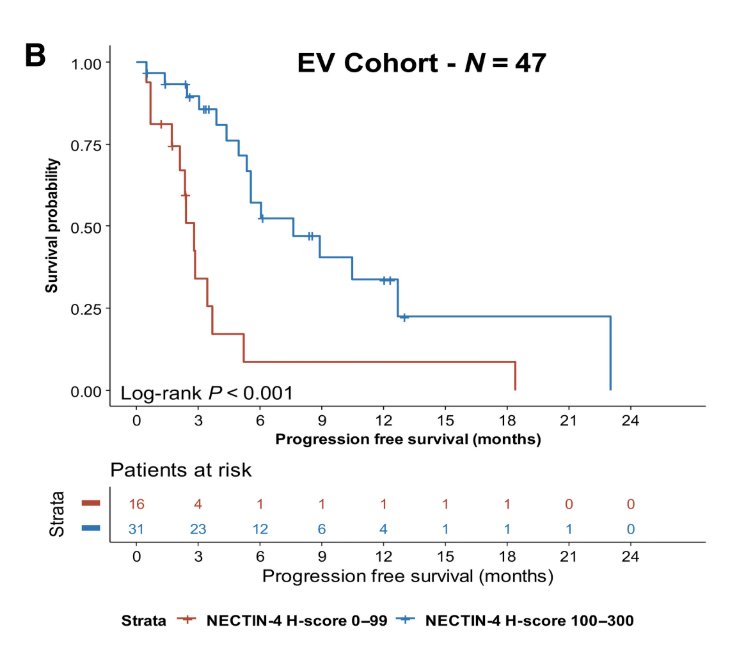
At ESMO 2024, Dr. Tom Powles showed in the EV-302 cohort that there was a consistent objective response rate benefit with enfortumab vedotin + pembrolizumab across all Nectin-4 H-score subgroups. Specifically, in patients with an H-score <275, the objective response rate was 63.6%, while in the H-score ≥275 subgroups, the objective response rate was 71.8%: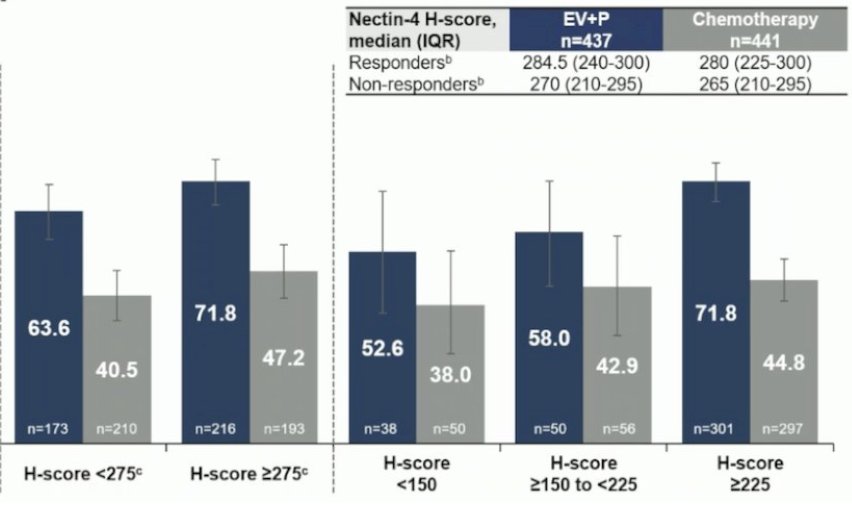
Dr. Klumper and Eckstein recently evaluated NECTIN4 amplifications as a genomic biomarker to predict enfortumab vedotin response in metastatic urothelial carcinoma.5 The found that NECTIN4 amplification represents a stable genomic alteration during metastatic progression and associates with enhanced membranous Nectin-4 protein expression: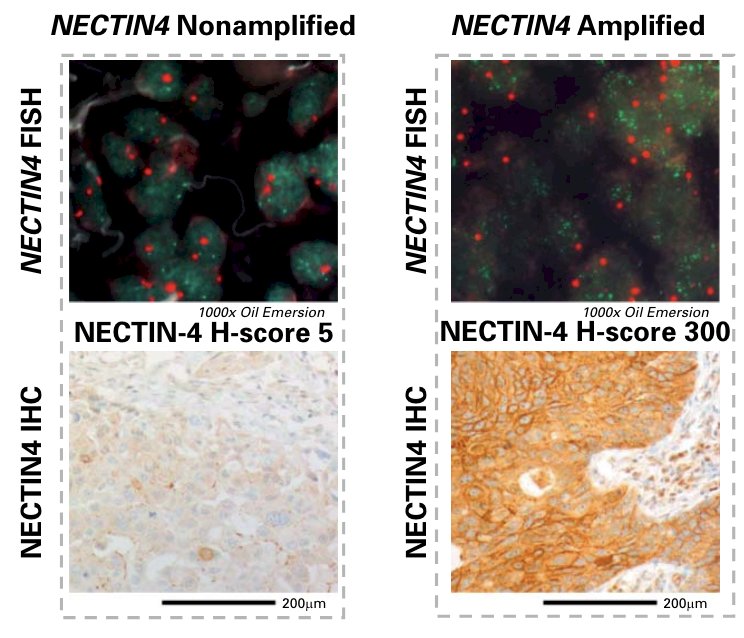
Overall, 96% of patients with NECTIN4 amplifications demonstrated objective responses to enfortumab vedotin compared with 32% in the non-amplified subgroup (p < 0.001). Nectin-4 amplification status was also associated with both prolonged progression-free survival and overall survival since enfortumab vedotin therapy started compared with non-amplified tumors: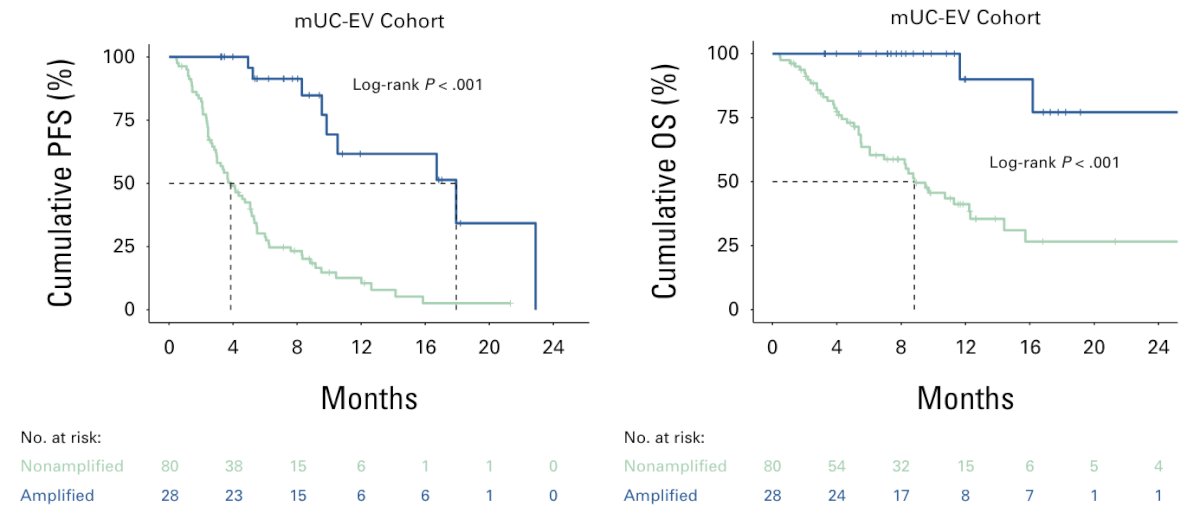
However, Nectin-4 amplification is not prognostic, as it was not associated with overall survival in non–enfortumab vedotin-treated metastatic urothelial carcinoma: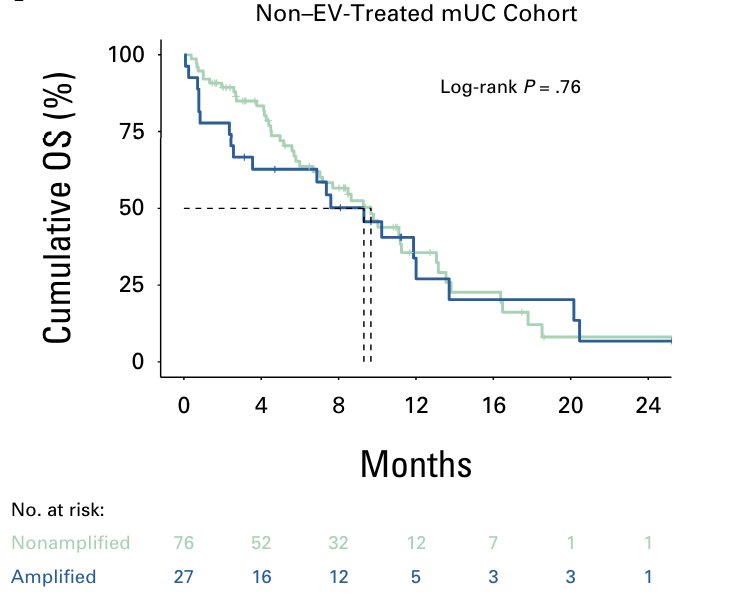
Regarding other antibody-drug conjugate targets, Dr. Eckstein discussed trastuzumab deruxtecan, which targets HER2. Trastuzumab deruxtecan was assessed in the Destiny-PanTumor02 trial of HER2-expressing tumors, with a 39% objective response rate among urothelial carcinoma patients, but up to 56.3% for IHC3+ patients. Disitamab vedotin is another investigational antibody-drug conjugate comprising a fully humanized HER2-directed monoclonal antibody. At ESMO 2024, Dr. Matt Galsky reported preliminary efficacy and safety of disitamab vedotin with pembrolizumab in the RC48G001 Cohort C trial. Disitamab vedotin confirmed objective response with the best percent change in the sum of diameters from baseline tumors, confirming response in both the HER2-positive and HER2-low groups: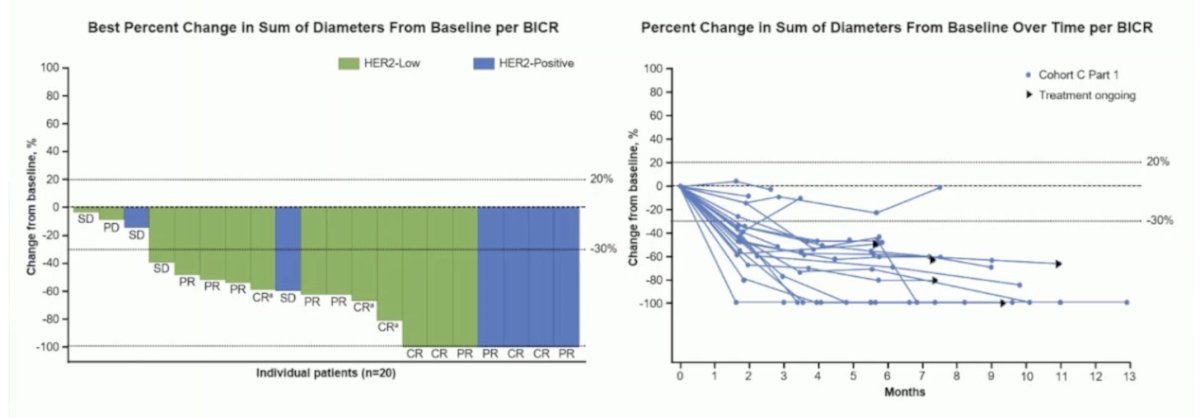
Finally, Dr. Eckstein noted that alterations of target proteins and payload resistance is a known mechanism of resistance for antibody-drug conjugates in other tumors (ie. Hodgkin lymphoma), as well as conventional chemotherapy, and small molecules (ie. TKIs).
Dr. Eckstein concluded his presentation discussing mechanisms of resistance to antibody-drug conjugates with the following take-home slide: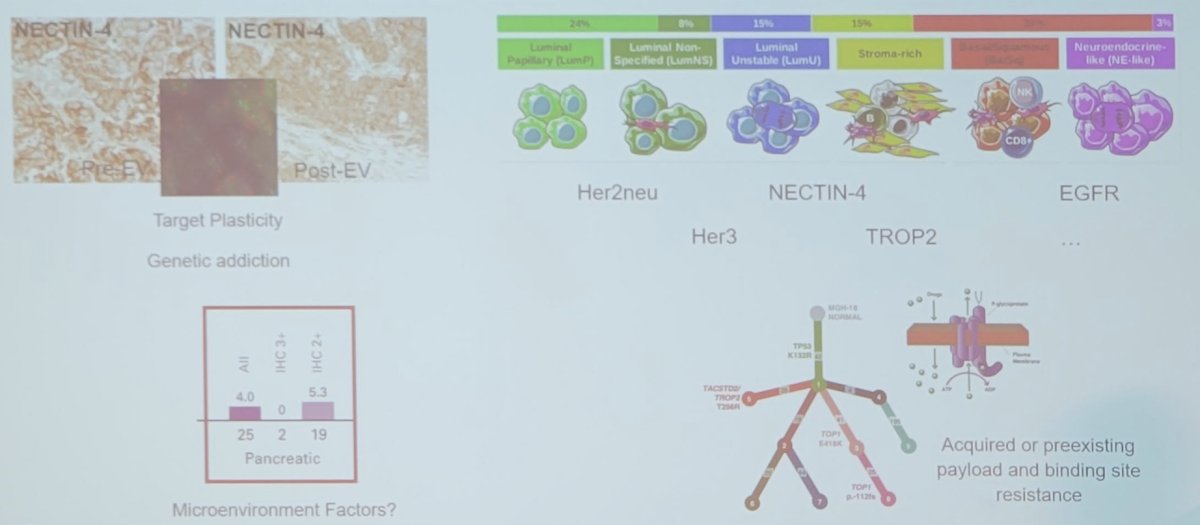
With regards to where we are going, specifically beyond the tremendous success of EV-302, he ended with a quote from Jacques Monod (June 17, 1976) “In science, self-satisfaction is death. Personal self-satisfaction is the death of the scientist. Collective self-satisfaction is the death of the research. It is restlessness, anxiety, dissatisfaction, agony of mind that nourish science.”
Presented by: Markus Eckstein, MD, Advanced Clinician Scientist, Friedrich Alexander University Erlangen-Nürnberg, Erlangen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 International Bladder Cancer Network (IBCN) Annual Meeting, Bern, Switzerland, Thurs, Sept 19 – Sat, Sept 21, 2024
References:- Rosenberg J, Sridhar SS, Zhang J, et al. EV-101: A Phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol 2020 Apr 1;38(10):1041-1049.
- Bahlinger V, Branz A, Strissel PL, et al. Associations of TACSTD2/TROP2 and NECTIN-4/NECTIN-4 with molecular subtypes, PD-L1 expression, and FGFR3 mutational status in two advanced urothelial cancer cohorts. Histopathology. 2024 Apr;84(5):863-876.
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10)875-888.
- Klumper N, Ralser DJ, Ellinger J, et al. Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin Cancer Res. 2023 Apr 14;29(8):1496-1505.
- Klumper N, Tran NK, Zschabitz S, et al. NECTIN4 Amplification is frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer. J Clin Oncol. 2024 Jul 10;42(20):2446-2455.


