(UroToday.com) The International Kidney Cancer Symposium 2021 annual hybrid meeting included a role of perioperative therapy in renal cell carcinoma (RCC) session and a presentation by Dr. Christopher Weight discussing the use of artificial intelligence for RCC diagnosis of CT scans. Dr. Weight notes that there has been substantial hype around artificial intelligence, and in 2016 Turing Aware Recipient Geoffrey Hinton stated that “People should stop training radiologists now. It’s just completely obvious that within 5 years, deep learning is going to do better than radiologists.”
There are three ways that CT segmentation can change the clinical practice of kidney cancer according to Dr. Weight:
- Increase diagnostic certainty and personalized prediction of cancer outcomes
- Predict the best surgical approach, perioperative complications and objectify patient counselling
- Predict post-operative renal function
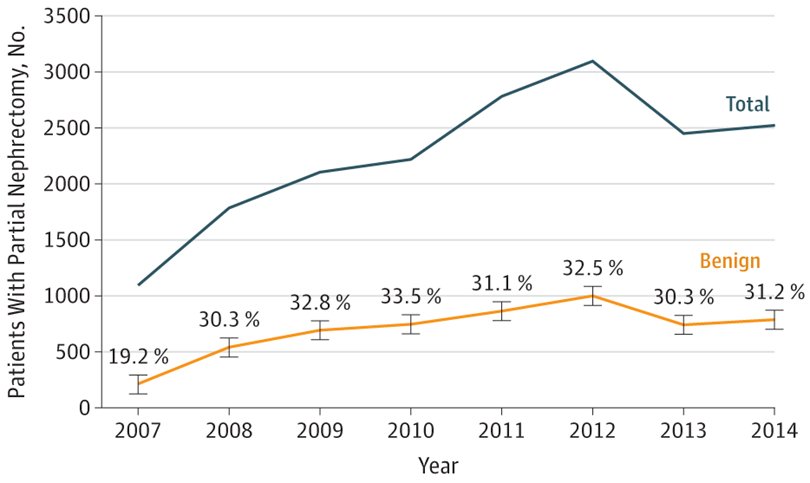
According to the Campbell-Walsh Urology textbook, the greatest clinical dilemma remains the inability to confidently differentiate between benign renal masses and RCC on clinical or radiographic testing. In a study using the Truven Health MarketScan Research Databases assessing 18,060 patients between 2007 and 2014, the overall prevalence of benign pathologic findings after partial nephrectomy was 30.9%.1 Furthermore, this incidence was affected by several factors including female sex, old age, and performance of CT imaging only as preoperative imaging modality. As follows is the annual prevalence of benign and malignant findings stratified by year in this study:
Dr. Weight emphasized that the utilization of artificial intelligence for RCC diagnosis all begins with segmentation, which is a computer vision task that separates a digital image into multiple parts. One aspect of this technology is creating a consistent, accurate, and reliable nephrometry score, which takes into account the nephron + tumor geometry. The nephrometry score has many uses, including prediction of:
- Surgical approach
- Operative times
- Complications
- Length of stay
- Pathologic stage
- Surgical margins
- Tumor growth rate
- Long-term renal function
- Cancer specific survival
- Overall survival
Work from Dr. Weight’s group assessed the reproducibility of the nephrometry scoring system among 95 patients treated surgically for a solid renal mass. Each renal tumor was separately scored by 6 reviewers, including 2 staff urologists, 1 staff radiologist, 2 trainees (1 urology, 1 radiology), and 1 medical student. Agreement in nephrometry score was substantial among the three staff physicians (0.72, 95% CI 0.64–0.80), and nephrometry score agreement continued to be substantial when including the trainees and medical student in the analysis (0.75, 95% CI 0.69–0.81). However, calculation of the nephrometry score requires unreimbursed time from very busy people (ie. radiologists, and surgeons). As follows is the algorithm for artificial intelligence generated segmentation, leading to code for translation of generated segmentations into fully automated nephrometry scores:

Automated RENAL scoring is just the beginning, with Dr. Weight noting that we can automate all nephrometry scores, PADUA, c-index, and contact surface area. Ultimately, we may be able to delve into new measurement metrics that humans cannot calculate that may be predictive. For example, Lubner and colleagues assessed whether CT texture features of newly diagnosed primary RCC correlated with pathologic features and oncologic outcomes.3 CT texture analysis was performed on large (>7 cm) untreated RCCs in 157 patients, with measures entropy, kurtosis, skewness, mean of positive pixels, and SD of pixel distribution histogram derived from a multiphasic CT using various filter settings:

When a coarse filter setting was used, entropy on portal venous phase CT images was positively associated with clear cell histologic findings (OR 134, 95% CI 16-1110) and was negatively associated with non-clear cell subtype findings. ROC curve analysis for entropy showed an AUC of 0.943 (95% CI, 0.892-0.993) for clear cell histologic findings, with similar values noted for non-clear cell histologic findings. The mean of positive pixels and the SD of the pixel distribution histogram were statistically significantly associated with histologic cell type in a similar fashion. Furthermore, there was a statistically significant association of texture features noted on unenhanced CT, including the SD of the pixel distribution histogram, the mean of positive pixels, and entropy, with the time to disease recurrence and death due to disease (HR 3.49, 95% CI 1.55-7.84).
What about using artificial intelligence to predict kidney function? Ye and colleagues4 evaluated the utility of parenchymal volume analysis for estimation of split renal function in patients with renal masses, given that split renal function are important for deciding about partial versus radical nephrectomy and assessing risk for developing severe chronic kidney disease after surgery. In this study, all patients (n = 374) with renal tumors and a normal contralateral kidney managed with partial nephrectomy (2010-2018), with preoperative/postoperative nuclear renal scans and cross-sectional imaging were analyzed. Parenchymal volumes were measured by free-hand scripting or software analysis. Parenchymal volumes estimated by free-hand scripting versus software analyses correlated strongly (r = 0.98, p < 0.001). Additionally, preoperative ipsilateral eGFR based on split renal function from parenchymal volume analysis versus nuclear renal scans also correlated strongly (r = 0.94, p < 0.001). Ipsilateral eGFR saved after partial nephrectomy correlated strongly with parenchymal volume preserved, however, the correlation was stronger when ipsilateral eGFRs were based on split renal function from parenchymal volume analysis rather than nuclear renal scans (z-statistic = 3.15, p = 0.002).
Dr. Weight concluded by emphasizing that ultimately his goal is to be in a position, with the utilization of artificial intelligence, that when a patient has a renal mass and undergoes a CT scan, that the information provided is the following:

Presented by: Christopher J. Weight, MD, MS, Center Director Urologic Oncology, Glickman Urologic and Kidney Institute, Cleveland Clinic, Cleveland, OH
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the International Kidney Cancer Symposium (IKCS) 2021 Annual Congress, November 5 and 6, 2021.
References:
- Kim JH, Li S, Khandwala Y, et al. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007-2014. JAMA Surg. 2019;154(3):225-231.
- Weight CJ, Atwell TD, Fazzio RT, et al. A multidisciplinary evaluation of inter-reviewer agreement of the nephrometry score and the prediction of long-term outcomes. J Urol. 2011;186:1223–1228.
- Lubner MG, Stabo N, Abel EJ, et al. CT Textural Analysis of Large Primary Renal Cell Carcinomas: Pretreatment Tumor Heterogeneity Correlates with Histologic Findings and Clinical Outcomes. Am J Roent 2016;207(1):96-105.
- Ye Y, Tanaka H, Wang Y, et al. Split renal function in patients with renal masses: Utility of parenchymal volume analysis vs nuclear renal scans. BJU Int 2020 May;125(5):686-694.


