The TIVO-3 trial provided data to support the FDA approval of Tivozanib among patients with relapsed or refractory advanced RCC, demonstrating significantly improved PFS over sorafenib (HR: 0.73, 95% CI, 0.56-0.95). Subsequent follow-up has shown that the PFS rate at 3-years is more than five times higher among those treated with tivozanib than with sorafenib (12% vs 2%, respectively). However, there has been no overall survival benefit demonstrated to date.

In this presentation, the authors provided an analysis of overall survival based on longer-term follow-up of these patients, highlighting the contribution of event accumulation and data maturation on the stability of KM survival estimates.
The authors used intention-to-treat analyses of the TIVO-3 cohort and performed Cox proportional hazards regression modeling to estimate the hazard ratio and 95% CI for OS l at prespecified (2-years after last-patient-in [LPI]; ≥251 events) and exploratory extended follow-up timepoints (≥270 events; database closure). All patients were followed for survival until death, consent withdrawal, or loss to follow-up.
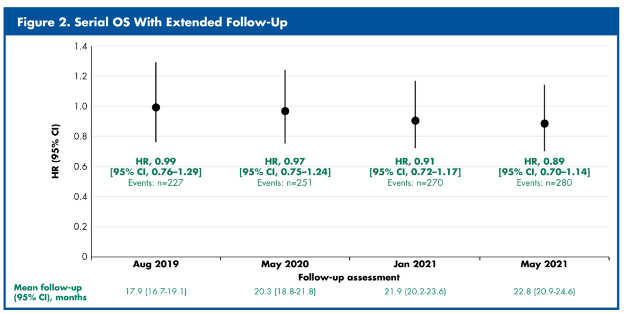
In TIVO-3, 350 patients were randomized 1:1 to receive either tivozanib (n=175) or sorafenib (n=175). As of August 2019, at 2-years following the last patient enrolled and with a mean follow-up of 17.9 months, 65% of patients experienced an event. However, there was no difference in overall survival evident (HR: 0.99, 95% CI, 0.76-1.29). Subsequent analyses are reported at 20.3 (May 2020), 21.9 (January 2021), and 22.8 (May 2021) months follow-up.
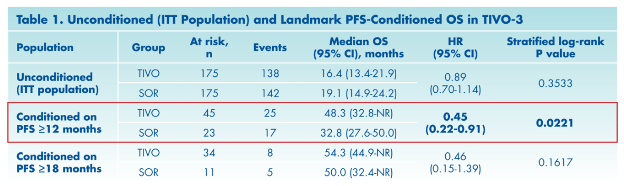
The authors performed a subsequent analysis of overall survival, conditioned on 12-month progression-free survival status. This analysis showed a greater benefit to tivozanib than was seen in the unconditioned analysis.
With the increasing follow-up to almost 23 months and realization of 80% of events, there has been a progressive decline in the hazard ratio for overall survival, favouring tivozanib.
Thus, Dr. Rini concluded that serial evaluation of overall survival in the TIVO-3 trial suggests early and consistent PFS benefit with TIVO over SOR is associated with an OS HR decline over time with more events, though without statistical significance.
Presented by: Brian Rini, MD, Professor of Medicine, Vanderbilt University Medical Center, Nashville, TN


