(UroToday.com) The second poster session at the 2022 International Kidney Cancer Symposium (IKCS): North America meeting featured a number of presentations, including one from Dr. Michael Atkins examining long-term progression-free survival outcomes for patients treated in the TIVO-3 randomized trial of tivozanib versus sorafenib.
The TIVO-3 trial provided data to support the FDA approval of Tivozanib among patients with relapsed or refractory advanced RCC, demonstrating significantly improved PFS over sorafenib in the primary independent review committee [IRC] analysis (HR:0.672, 95% CI: 0.52-0.87). In this presentation, the authors provided longer-term follow-up of these patients. Long-term survival without disease progression (LT-PFS) is a clinically meaningful outcome among patients with advanced mRCC who have received two or more lines of prior therapy. Thus, at 6-month intervals up to 4 years following initial study medication, the authors examined the proportion of TIVO-3 patients living progression-free with relapsed or refractory mRCC.
The authors relied upon investigator assessment of disease progression to calculate long-term survival without disease progression up to a data cut off of May 24, 2021. They then compared landmark values of LT-PFS at 6, 12, 18, 24, 30, 36, 42, and 48 months between patients who received tivozanib and those who received sorafenib. Results include the ITT population, with censoring for missing assessments and discontinuation without PD. They used Cox proportional hazards and log-rank statistics to estimate the HR and 95% CI for INV-PFS with odds ratios (ORs) reported for landmark timepoints up to 36-months. Additionally descriptive subgroup analyses were performed.
As has previously been reported, 350 patients were randomized to tivozanib (n=175) or sorafenib (n=175) within the context of TIVO-3. Overall, the authors demonstrated that progression-free survival was superior among those who received tivozanib (HR: 0.624, 95% CI: 0.49-0.79). Examining landmark LT-PFS rates up to 48-months, these were consistently higher among patients receiving tivozanib, compared to sorafenib: 12% vs 2% and 7.6% vs 0% at 3- and 4 years, respectively.
Further, despite low numbers of patients at risk with extended follow-up, the authors were able to identify subgroups of patients who, when treated with tivozanib, had LT-PFS at 3 years exceeding 15% including those with IMDC favorable risk, female gender, ECOG PS0, age ≥65 years, and region North America.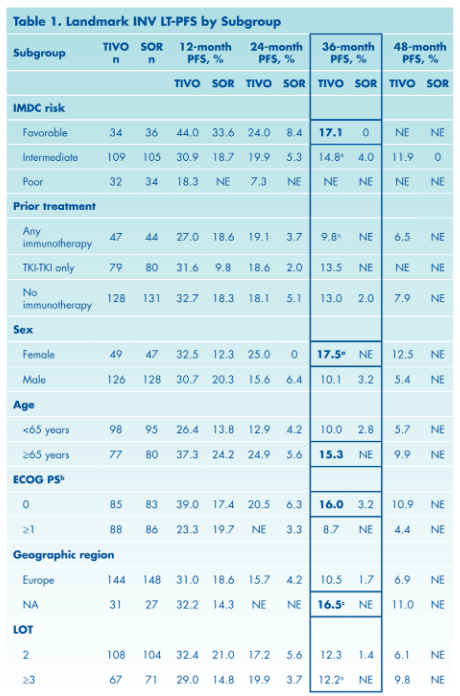
Thus, the authors conclude that the long-term data from TIVO-3 are consistent with the primary report and support the role of tivozanib in this patient population.
Presented by: Michael Atkins, MD, Georgetown University Medical Center, Washington DC


