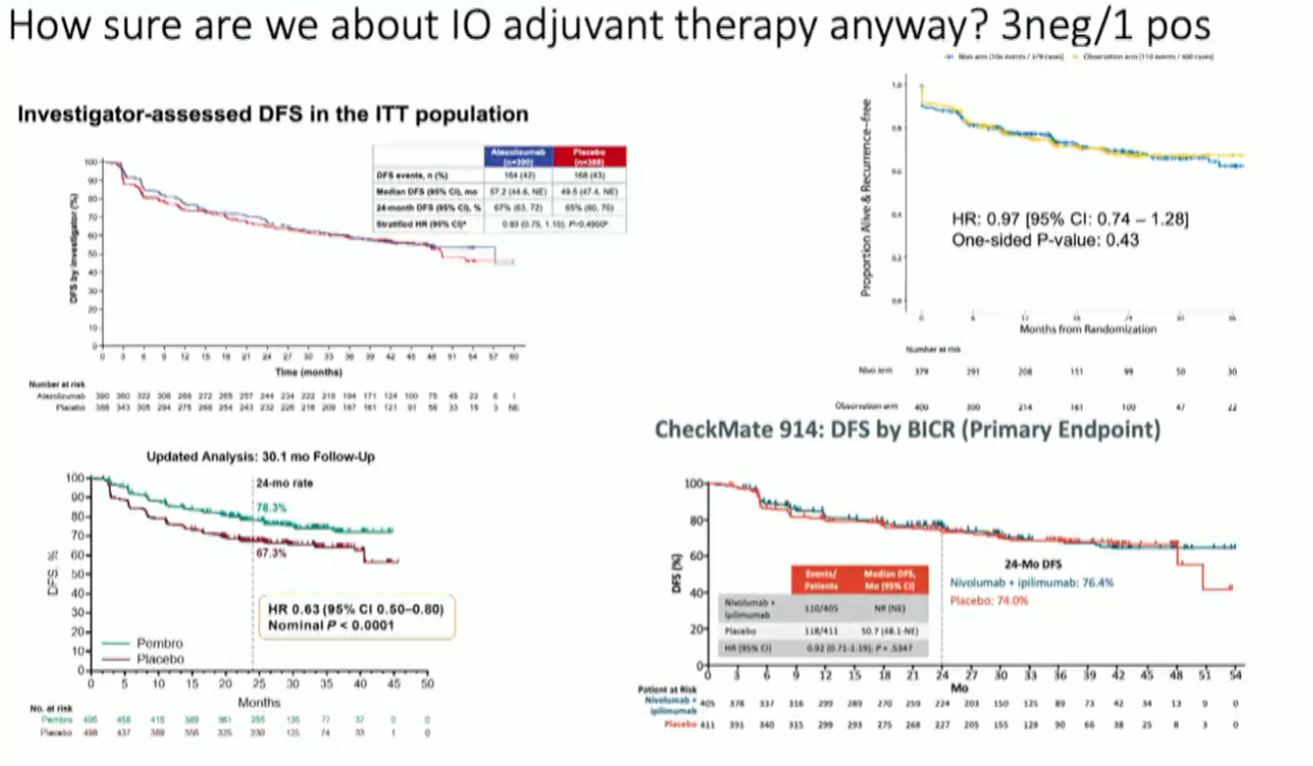
(UroToday.com) While adjuvant pembrolizumab has demonstrated an improvement in progression-free survival in high-risk resected renal cell carcinoma (RCC), excitement has somewhat dampened in light of subsequent negative adjuvant IO trials.
Dr. Vitaly Margulis presented a case of a patient with resected 10.5cm pT3b ccRCC, grade 4 with 5% sarcomatoid differentiation. Surgical margins were negative. The question posed to the debaters was whether or not to offer adjuvant pembrolizumab.
Dr. Bradley McGregor spoke in favor of adjuvant therapy. He began by summarizing the available adjuvant data across all trials anti-VEGF TKIs.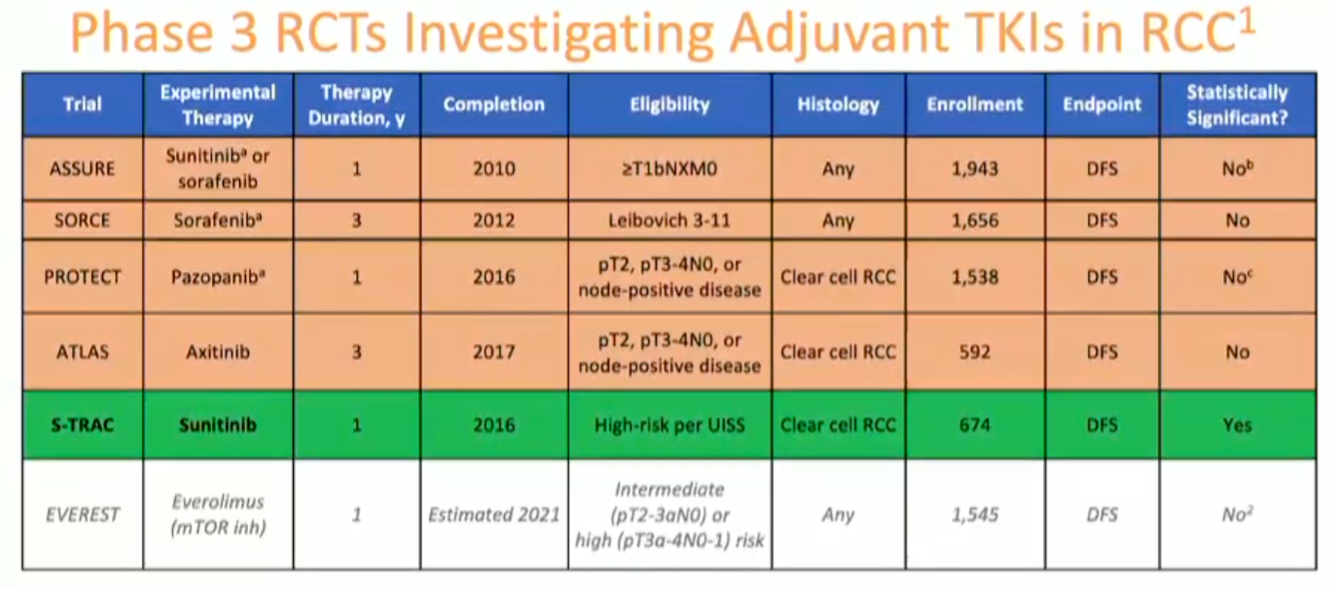
He noted the positive S-TRAC trial of adjuvant sunitinib demonstrating a statistically significant improvement in recurrence-free survival. However, there was no demonstrated overall survival benefit, and toxicity can be considerable, thus leading to very low uptake in clinical practice.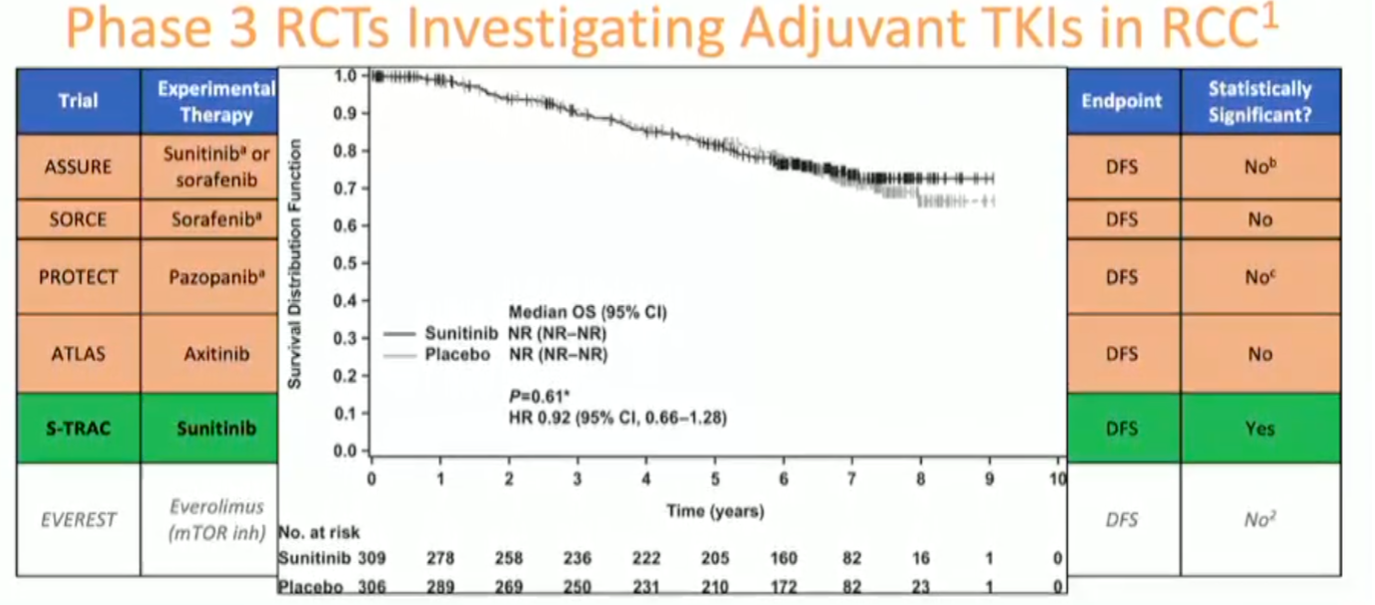
He next moved on to discuss the design of Keynote-564, the randomized phase III trial of hadjuvant pembrolizumab versus placebo in high-risk resected ccRCC. He specifically pointed to the inclusion criteria: pT2 high grade, pT3+ any grade, pN1 (only 8% of the cohort), or M1 resected NED (only 6% of the cohort).
With longer follow-up, the disease-free survival benefit of pembrolizumab is maintained at 30 months. The treatment and control arm curves separate early ~3-9 months and remain separated. 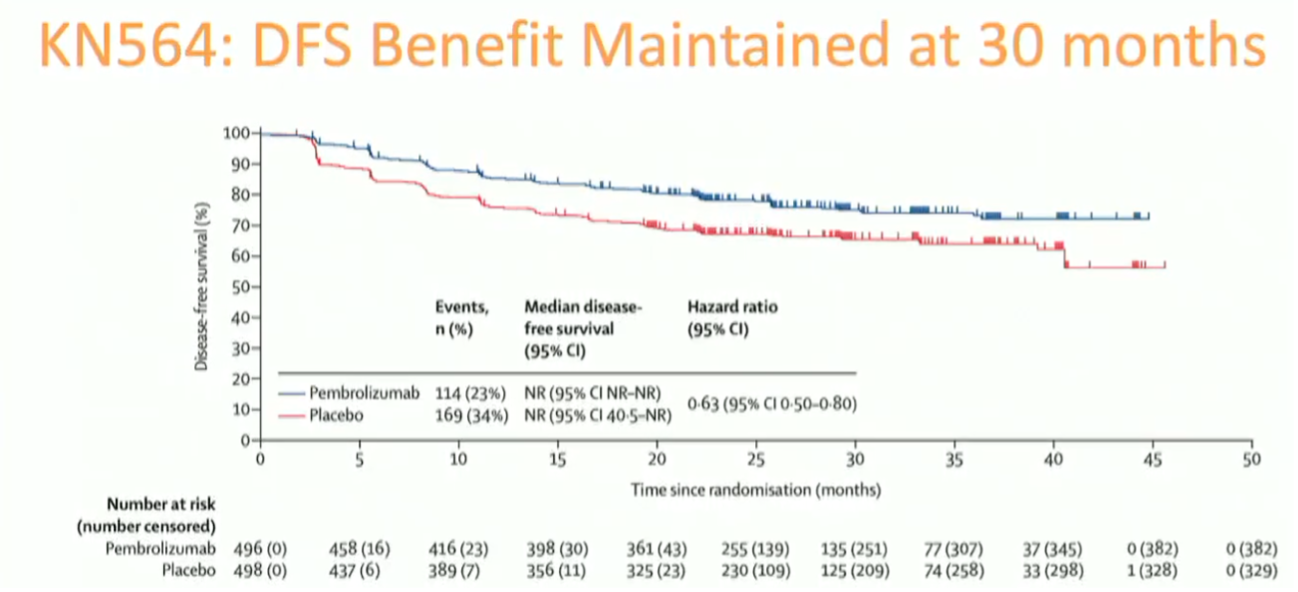
The benefit of adjuvant pembrolizumab was observed across clinical subgroups, with high-risk and M1 NED cohorts deriving the greatest benefit from adjuvant immunotherapy.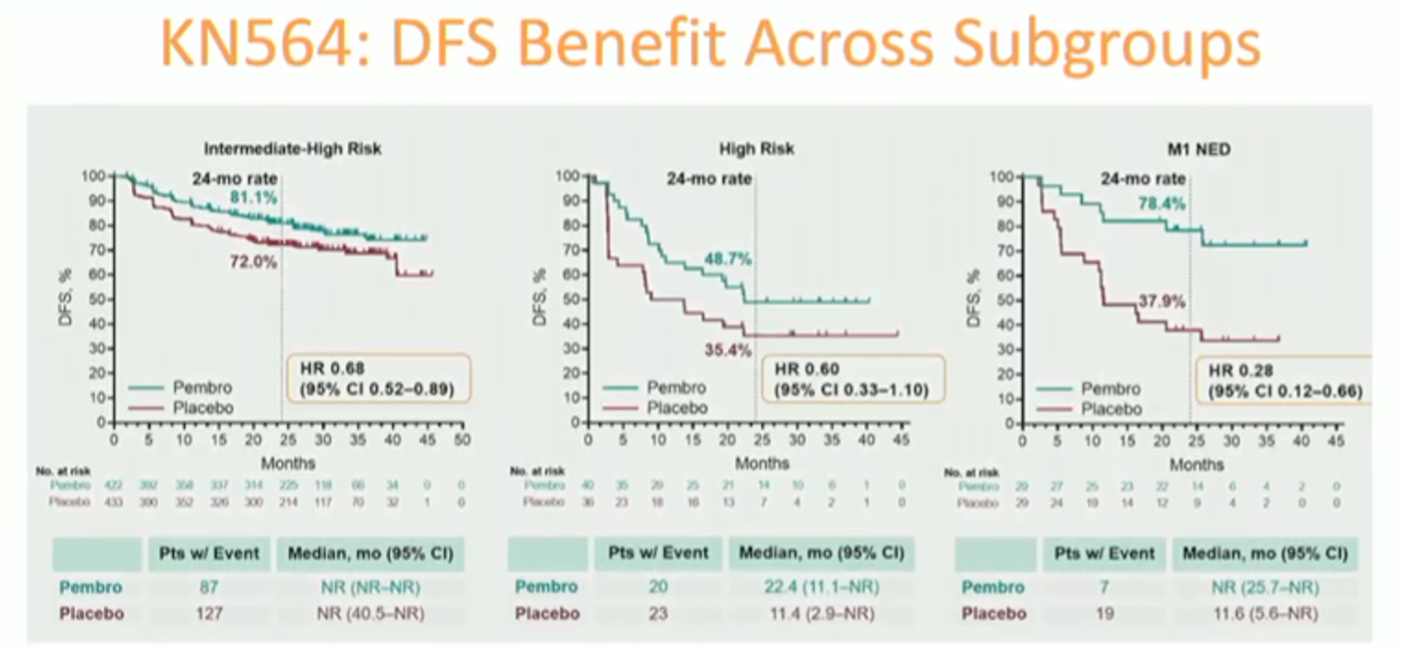
Dr. McGreggor addressed the utility of disease-free survival as a primary endpoint for adjuvant trials in resected RCC. Based upon the high-quality epidemiologic data supporting a very strong correlation between PFS and OS, he indicated that PFS is a very reasonable endpoint for adjuvant RCC trials.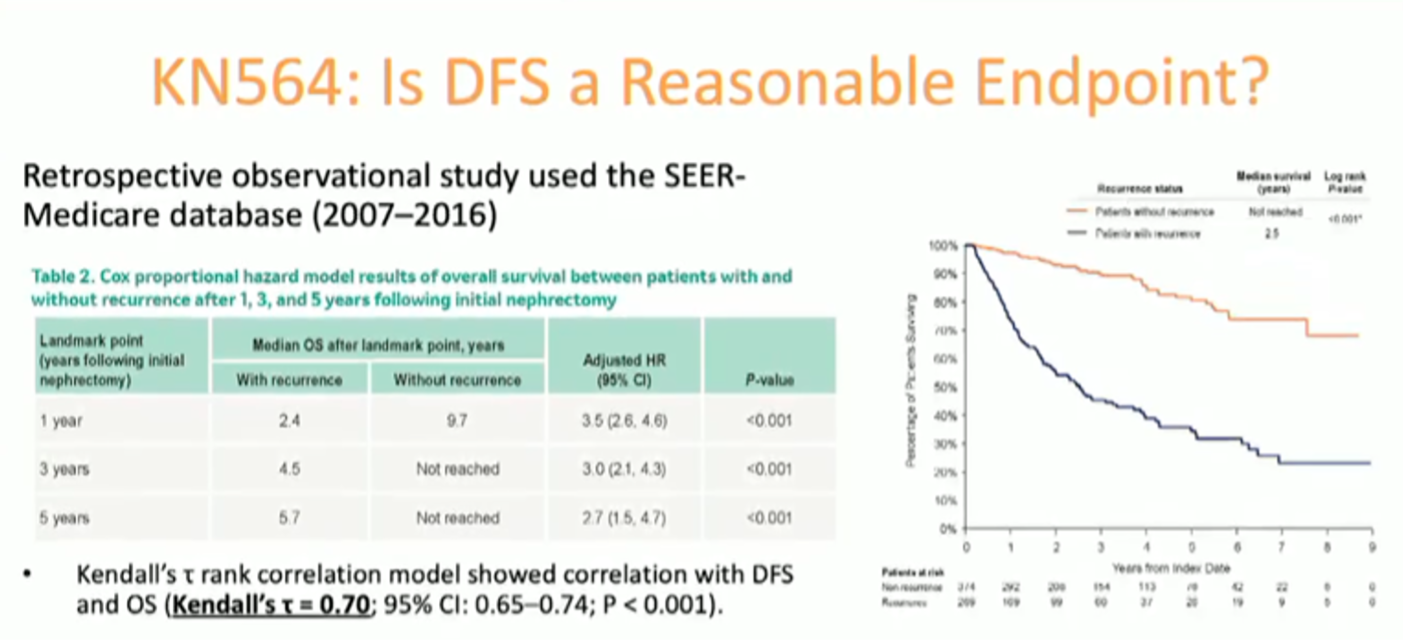
While the OS signal from Keynote-564 is encouraging, the number of survival events is, to date, relatively low, and conclusion cannot yet be drawn regarding an overall survival benefit with adjuvant pembrolizumab.
Addressing the discordant results from other adjuvant trials of anti-PD-1/PD-L1 ICIs, Dr. McGregor emphasized the differences in the various study populations as one possible reason for the divergent outcomes.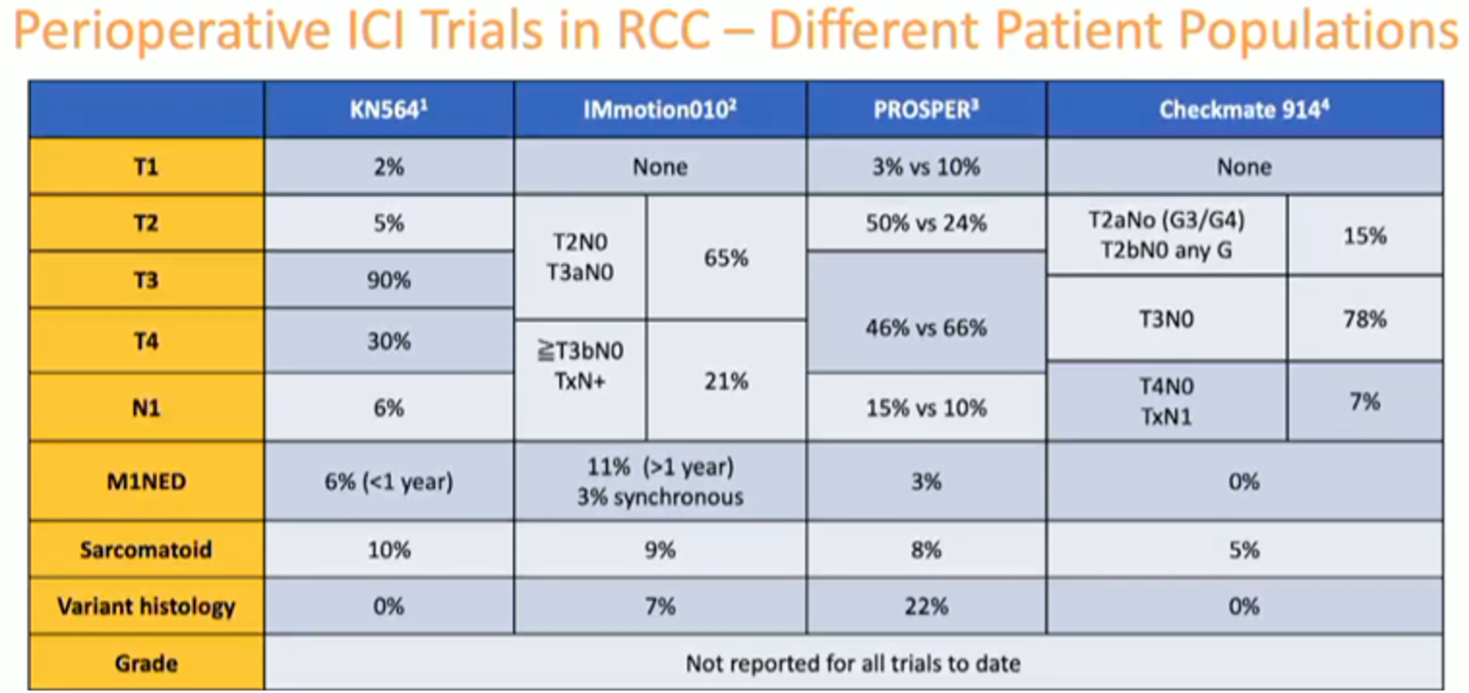
Ultimately, he suggested, we need better predictive biomarkers to identify patients who stand to benefit from adjuvant systemic therapy and those who do not. He highlighted the promise of cfDNA as a predictive/prognostic biomarker in resected muscle-invasive urothelial cancer though the sensitivity of the assay was somewhat limited in the MIBC study and RCC is generally a low DNA shedding tumor type.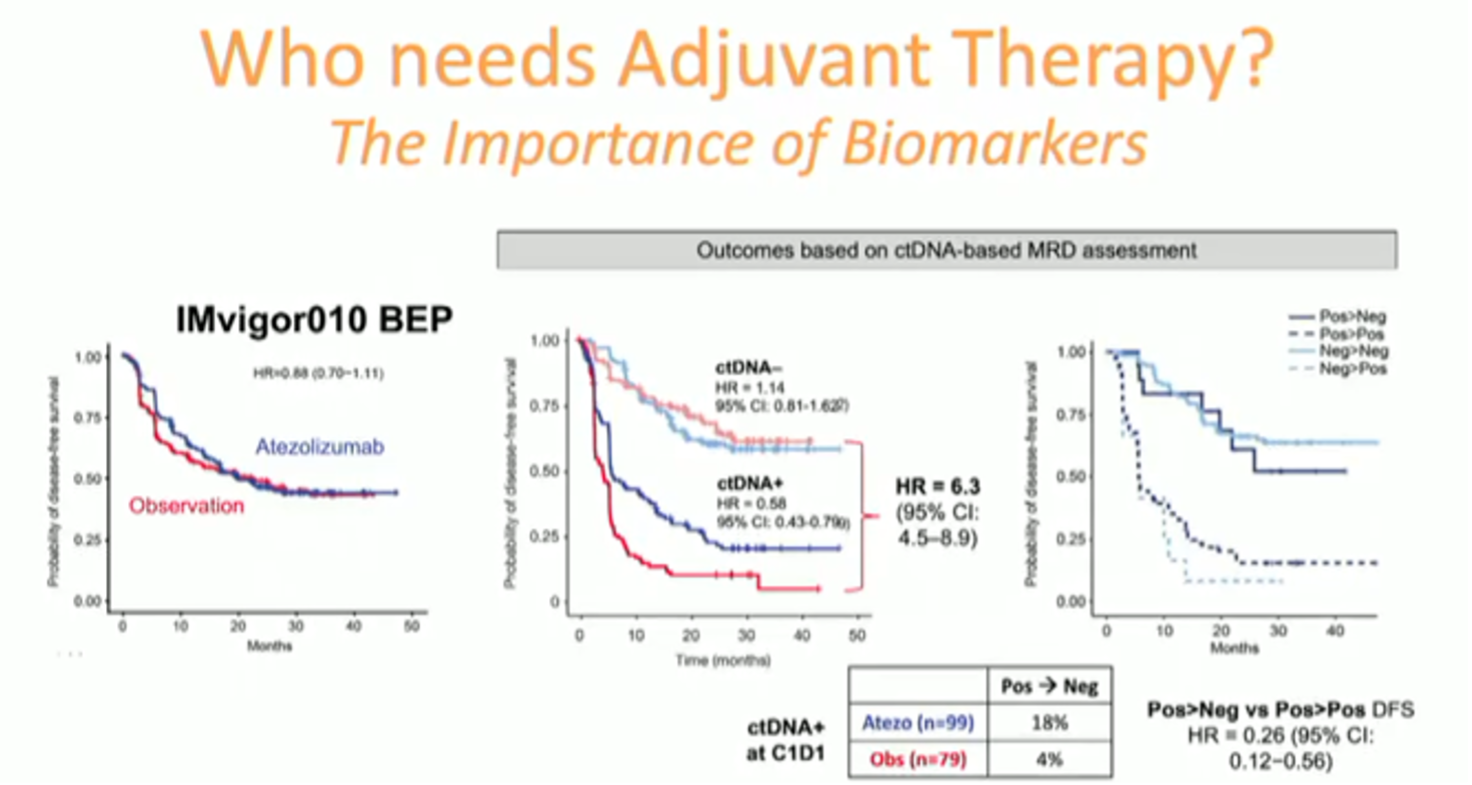
He also suggested that the difference in outcomes across adjuvant IO trials could be related to different properties among the specific immune checkpoint inhibitors, themselves.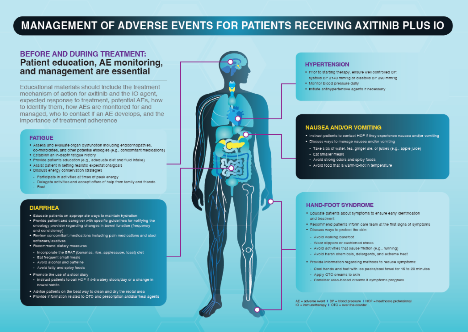
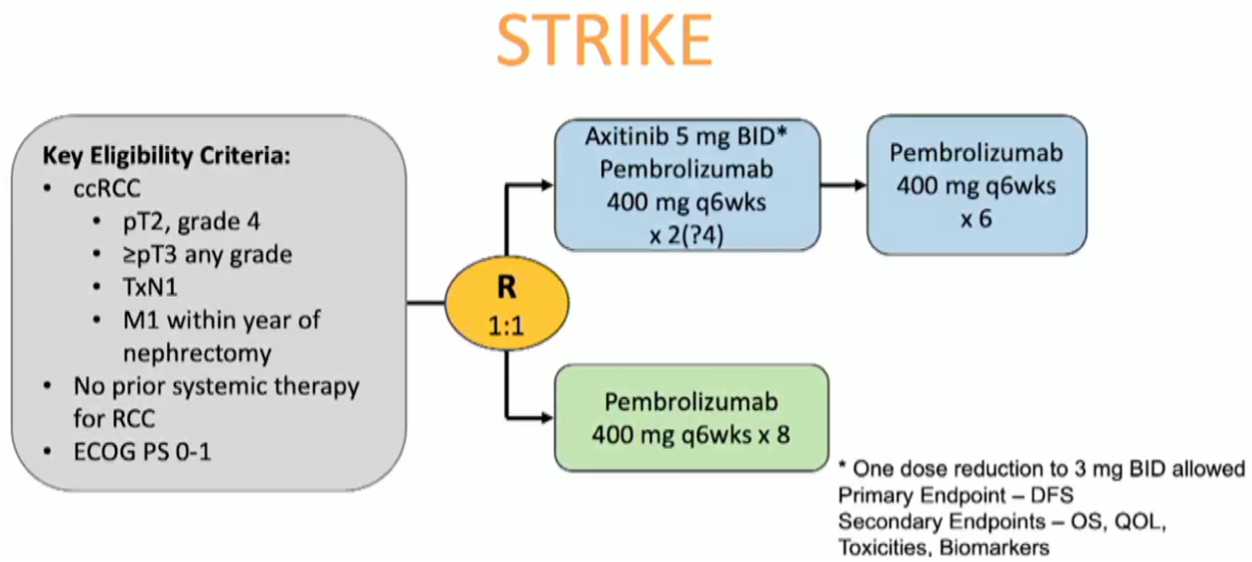
Dr. McGregor concluded by highlighting ongoing trials seeking to augment the disease-free survival benefit of pembrolizumab via combination with axitinib or with belzutifan.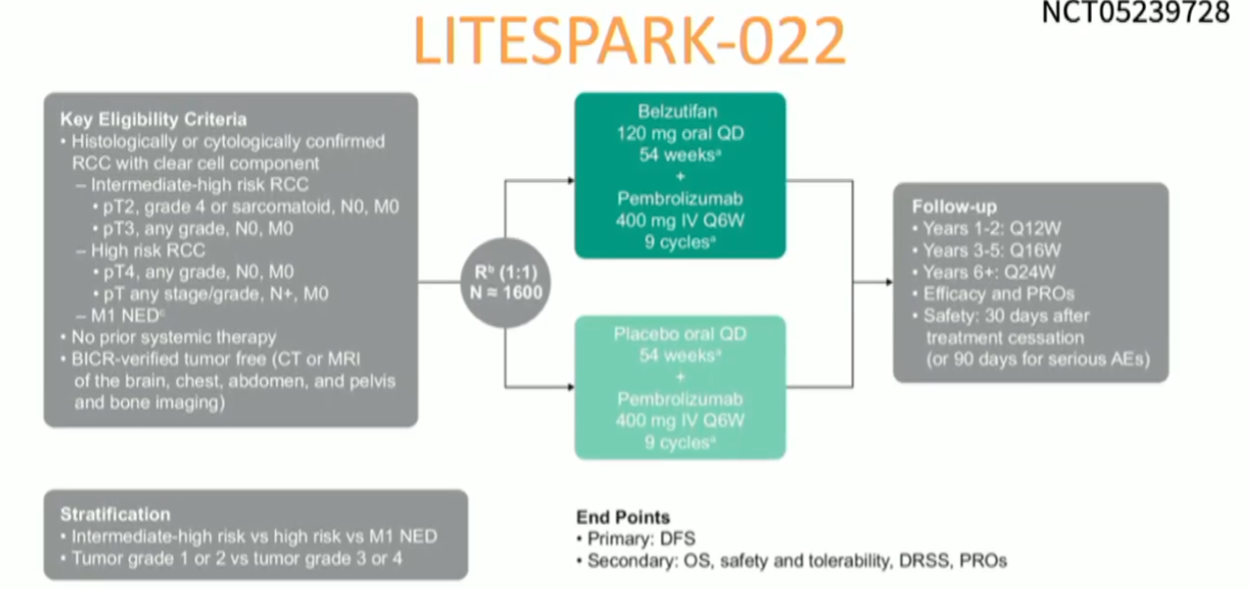
Dr. Naomi Haas next presented the case against adjuvant immunotherapy. She began by highlighting the incomplete utility of the presently-available UISS risk stratification, with up to ~20% of nominally ‘low-risk’ patients developing recurrent disease and ~30% of ‘high-risk’ patients never developing recurrent disease. Ultimately, she indicates, the lack of rigorously validated prospective biomarkers in resected RCC limits the development of adjuvant systemic therapies and leads to over- and under- treatment of different patients.
Dr. Haas moved on to highlight the significant toxicity associated with adjuvant anti-VAGF TKIs with ~60% grade 3 or higher adverse events in each trial.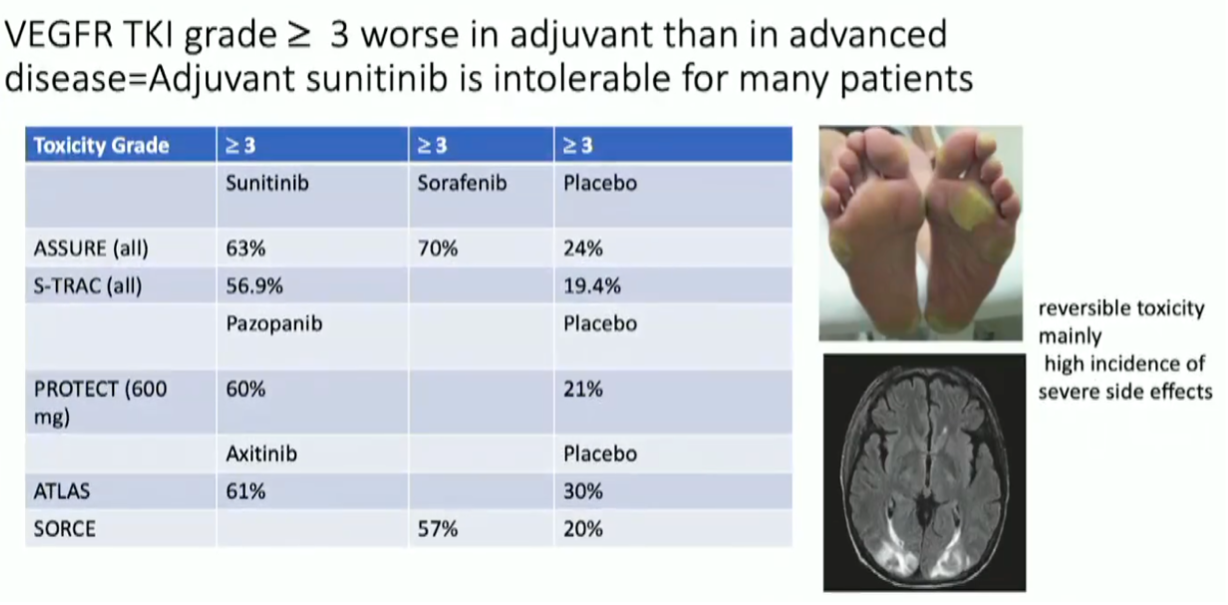
While anti-PD-1 immunotherapy is certainly better-tolerated for most patients, the risk of irreversible and severe and life-threatening adverse events, while small, is not negligible. Certainly the cases of severe immune-related adverse events like encephalitis, hypophysitis, and myocarditis should not be ignored when considering adjuvant systemic therapy in this clinical context. Furthermore, grade 3+ adverse events lead to discontinuation of adjuvant pembrolizumab in nearly 20% of the cohort.
Presented by:
- Vitaly Margulis, MD, UT Southwestern Medical Center, Dallas, TX
- Bradley McGregor, MD, Dana-Farber Cancer Institute, Boston, MA
- Naomi Haas, MD, Hospital of the University of Pennsylvania, Philadelphia, PA


