(UroToday.com) The 2022 IKCS North American annual meeting featured an Academy of Kidney Cancer Investigators session with a presentation by Dr. Wenxin Wu discussing preliminary findings from his laboratory-based investigation seeking to develop circulating biomarkers for the management of renal cell carcinoma (RCC).
While a number of potential circulating biomarkers have been proposed, including: cytokines, circulating tumor cells, circulating tumor DNA, proteins, and metabolites, none thus far has been rigorously validated. Cell-free methylated DNA immunoprecipitation sequencing (cfMeDIP-seq) is a novel approach to augment the signal-to-noise ratio of the more conventional cfDNA approach which Dr. Wu proposes to use for subsequent biomarker development. In addition, KIM-1 is a renal injury molecule overexpressed in tumors derived from proximal renal tubule cells. Specifically, plasma KIM-1 has been shown to decrease following nephrectomy and a subset of patients exhibit ongoing detection of KIM-1.
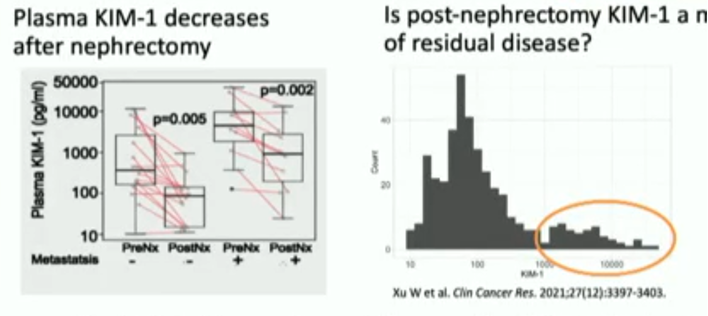
Furthermore, preliminary data from patient plasma derived from the S-TRAC trial of adjuvant sunitinib in resected RCC demonstrates that post-nephrectomy KIM-1 is prognostic for progression-free survival (PFS) and overall survival (OS).

Utilizing patient-derived plasma, Dr. Wu has sought to validate both cfMeDIP-seq as well as serum KIM-1 as circulating biomarkers in RCC. If validated, future studies will focus on using these biomarkers to prospectively identify:
1. Patients with renal masses for active surveillance versus surgery
2. Patients deriving the greatest benefit from adjuvant pembrolizumab in resected RCC
3. The capacity for circulating KIM-1 to serve as an early biomarker of response/resistance to systemic therapy in the metastatic setting
Presented by: Wenxin (Vincent) Xu, MD, Medical Oncologist, Dana Farber Cancer Institute


