The 2024 Southern California Genitourinary Cancer Research Forum featured a kidney cancer session and a RCC case-based trial discussion focusing on localized RCC, front-line RCC, salvage RCC, and non-clear cell RCC. Moderator Dr. Monty Pal started by emphasizing the following localized kidney cancer trials available in Southern California:

The first trial in the localized kidney cancer space highlighted in detail by the panel was the PROBE trial, a phase III trial of immunotherapy-based combination therapy with or without cytoreductive nephrectomy for metastatic renal cell carcinoma. The primary outcome for this trial is overall survival and secondary outcomes include overall survival between arms and complications of surgery: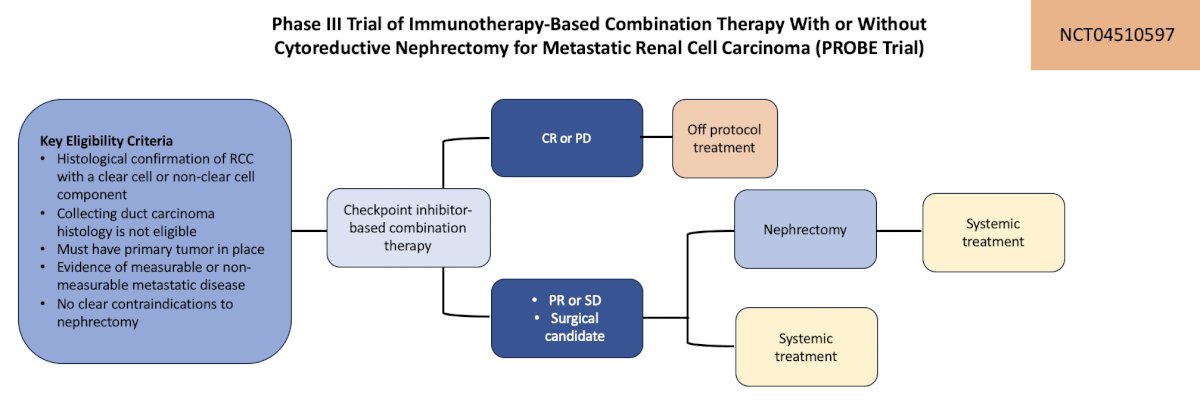
This trial is accruing at Cedars Sinai Medical Center, City of Hope, and USC.
The second trial in the localized kidney cancer space highlighted by the panel was the SAMURAI trial, a randomized phase II trial of stereotactic ablative radiation therapy for metastatic unresected renal cell carcinoma receiving immunotherapy. Stratification factors include: IMDC intermediate versus poor risk, planned immunotherapy (IO-IO versus IO-VEGF), and histology (clear cell versus non-clear cell). The primary outcome is nephrectomy and radiographic progression free survival, and secondary outcomes include:
- Objective response rate
- Radiographic progression free survival
- Rate of cytoreductive nephrectomy
- Treatment-free survival
- Overall survival
The trial design for SAMURAI trial design is as follows: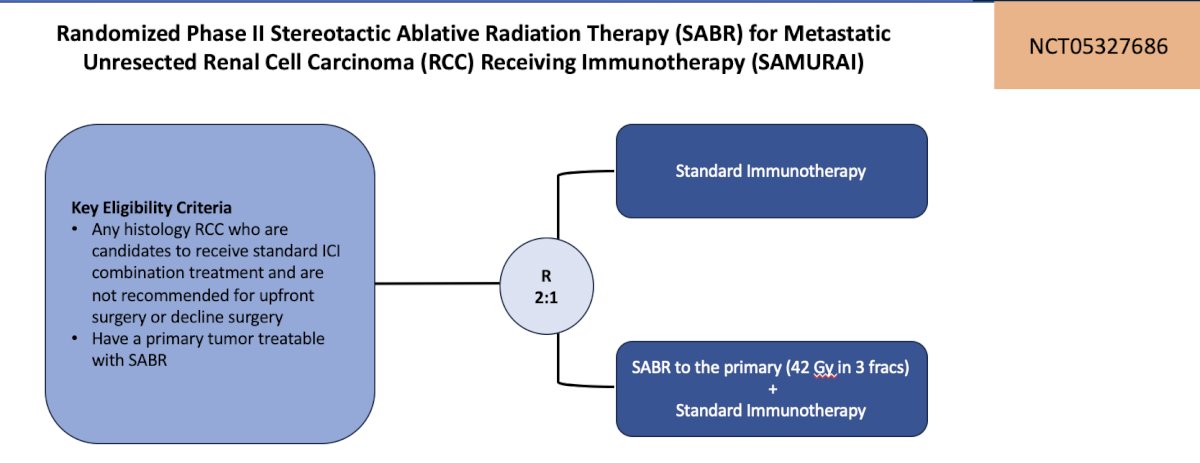
This trial is accruing at UC San Diego.
The third trial in the localized kidney cancer space highlighted by the panel was a prospective study of interstitial brachytherapy for unresectable/unablatable T1/T2a renal masses. The primary outcome is safety, feasibility, and local control, and secondary outcomes include treatment response of primary tumor, 12-month distant progression free survival. The trial design is as follows: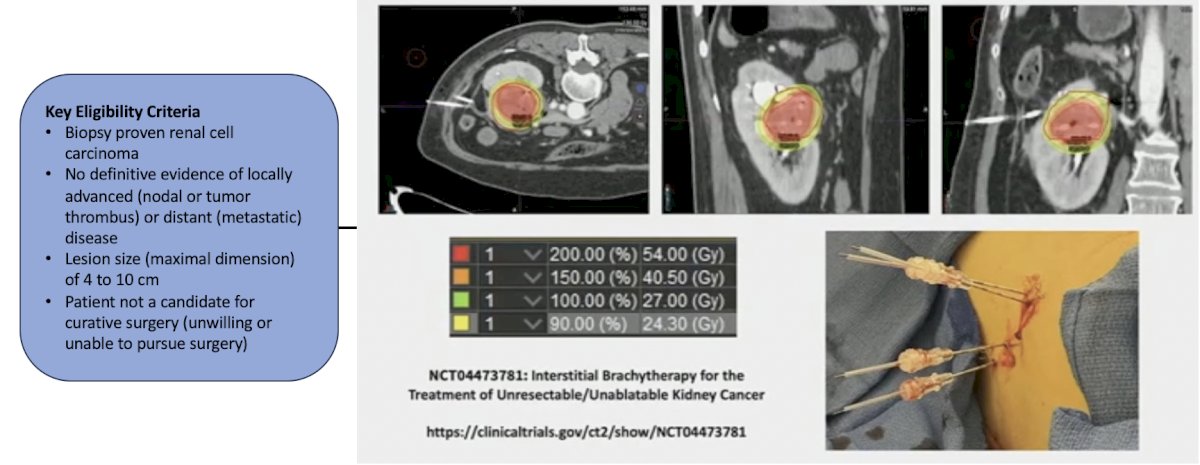
This trial is accruing at UCLA.
Dr. Pal then emphasized the following trials available in Southern California in the first-line metastatic RCC setting: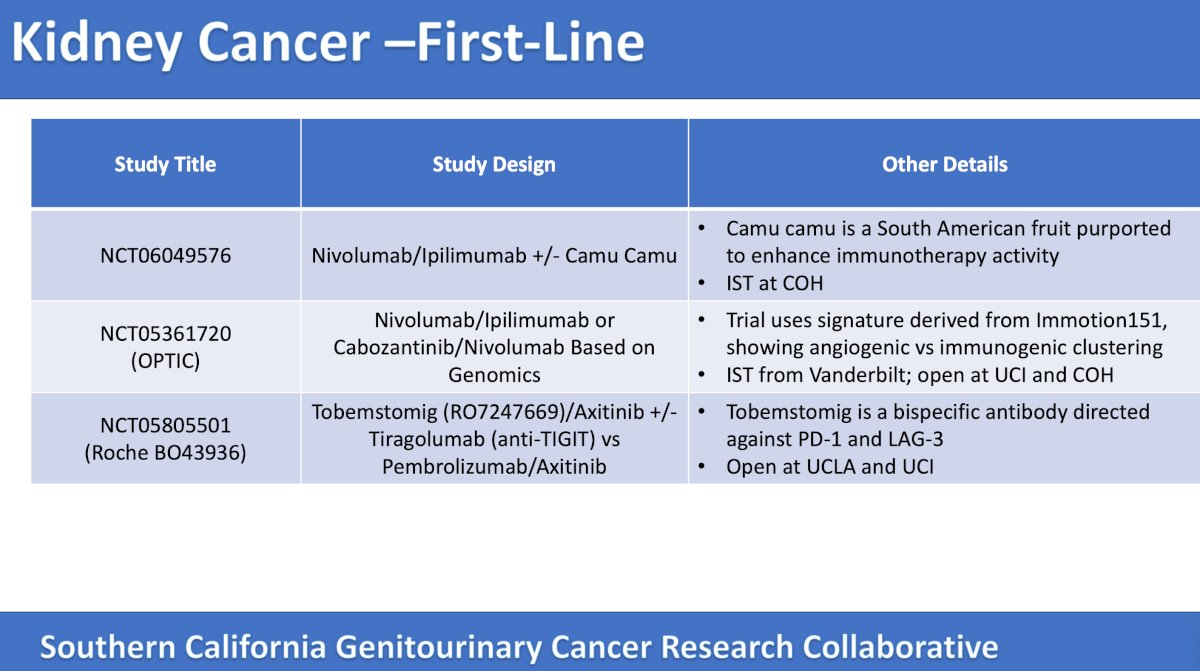
The first trial highlighted by the panel in the first-line metastatic RCC setting was a combination of nivolumab + ipilimumab with and without camu camu. Patients are to be randomized 2:1 to nivolumab + ipilimumab + camu camu versus nivolumab + ipilimumab, with a primary outcome of Ruminococcus abundance in stool samples. Secondary outcomes include clinical efficacy, safety, gut microbiome diversity, and function. The trial design for this trial is as follows: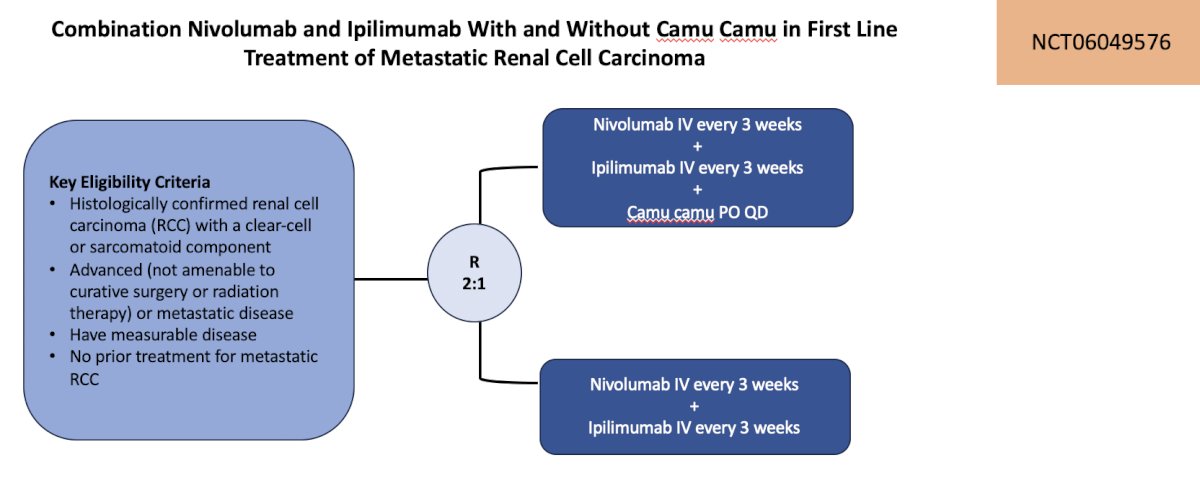
This trial is accruing at City of Hope.
The second trial highlighted by the panel in this disease space was the OPTIC RCC Study, a trial of genetic testing to select therapy for the treatment of advanced or metastatic kidney cancer. After RNAseq, Cluster 1/2 will receive nivolumab every 4 weeks + cabozantinib daily and those with Cluster 4/5 will receive nivolumab every 3 weeks + ipilimumab every 3 weeks. The primary outcome is objective response rate per RECIST, and secondary outcomes are PFS and depth of response > 80% at 6 months. The trial design of the OPTIC RCC Study is as follows: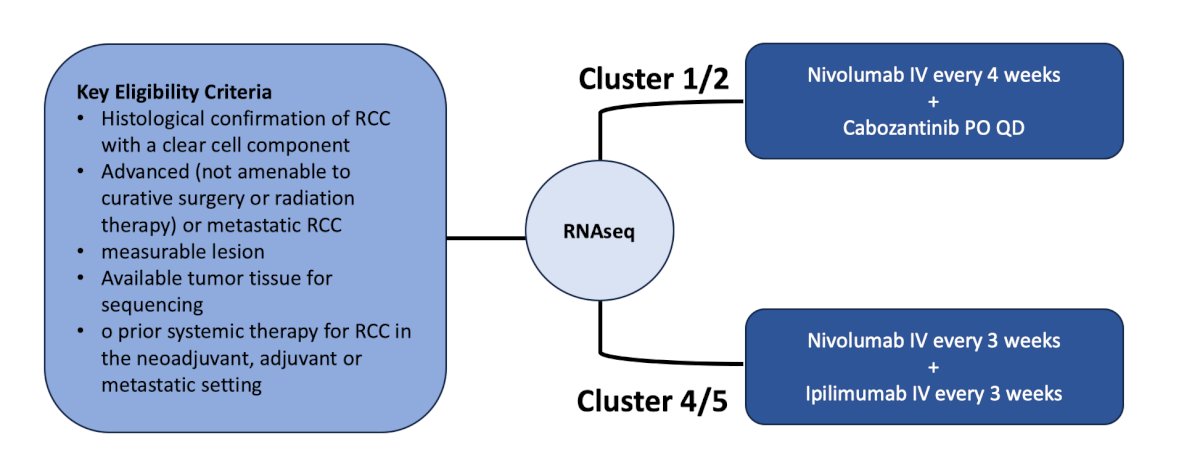
This trial is accruing patients at City of Hope and UC Irvine.
The third trial highlighted by the panel in this disease space was a randomized open label phase II study of immune checkpoint inhibitor combinations with axitinib in patients with previously untreated locally advanced unresectable or metastatic renal cell carcinoma. Patients will be randomized 1:1:1 to tobemstomig (mechanism of action: bispecific antibody directed against PD-1 and LAG-3) + axitinib versus tobemstomig + tiragolumab (mechanism of action: anti-TIGIT) + axitinib versus pembrolizumab + axitinib. The primary outcomes are PFS and safety, and the secondary outcomes are overall survival, objective response rate, and duration of response. The trial design is as follows: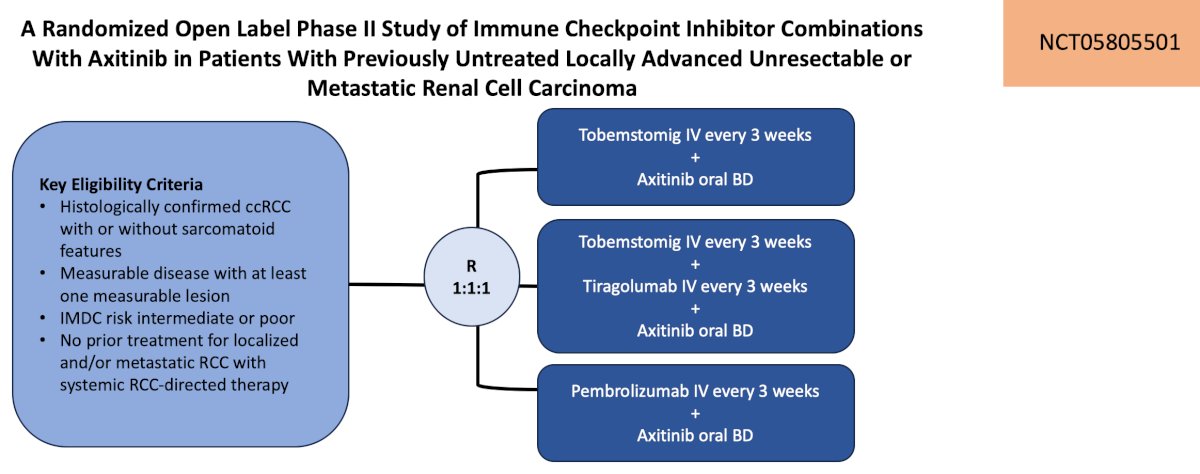
This trial is accruing at UCLA and UC Irvine.
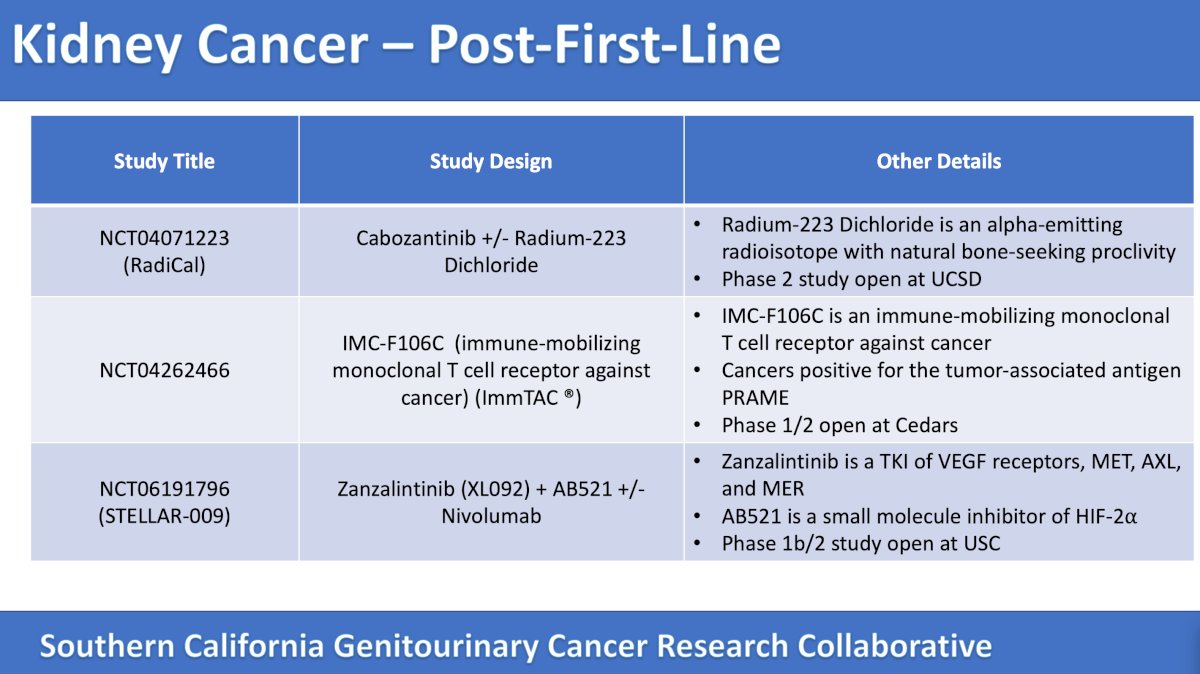
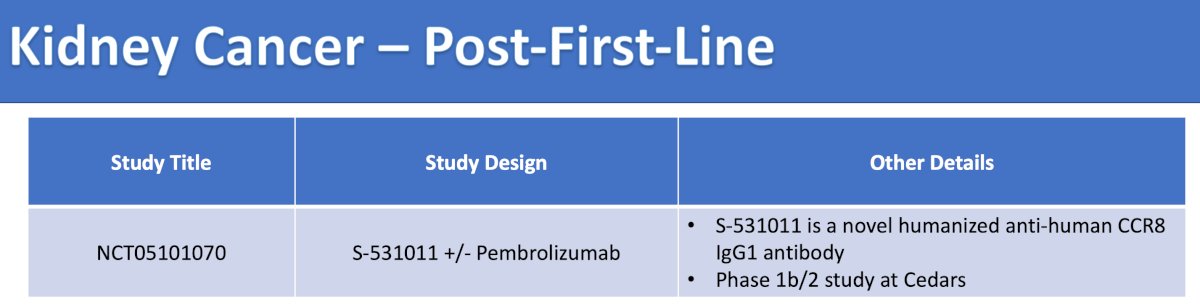
There are many trials in Southern California available in the post first-line setting for RCC as highlighted in the following tables: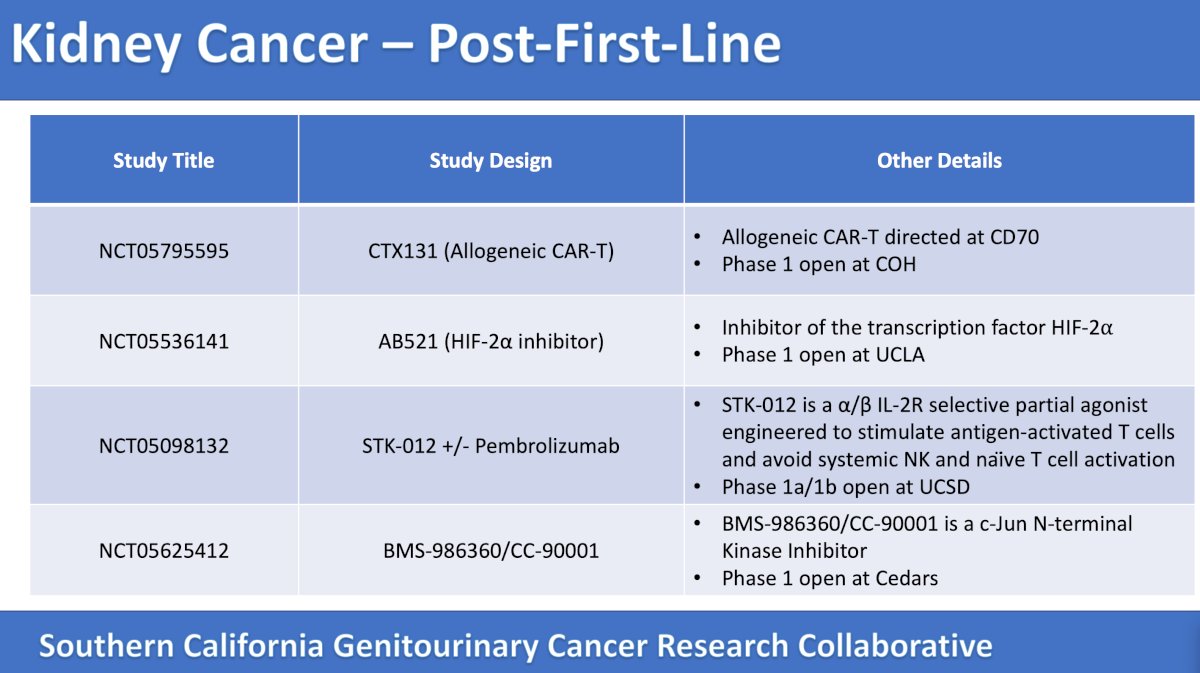
The first trial in the post first-line setting discussed in detail by the panel was a phase 1/2 open label, multicenter, dose escalation and cohort expansion study of the safety and efficacy of anti-CD70 allogeneic CRISPR-Cas9-Engineered T Cells (CTX131) in adult patients with relapsed or refractory solid tumors. The trial design for this study, which is accruing patients at City of Hope, is as follows: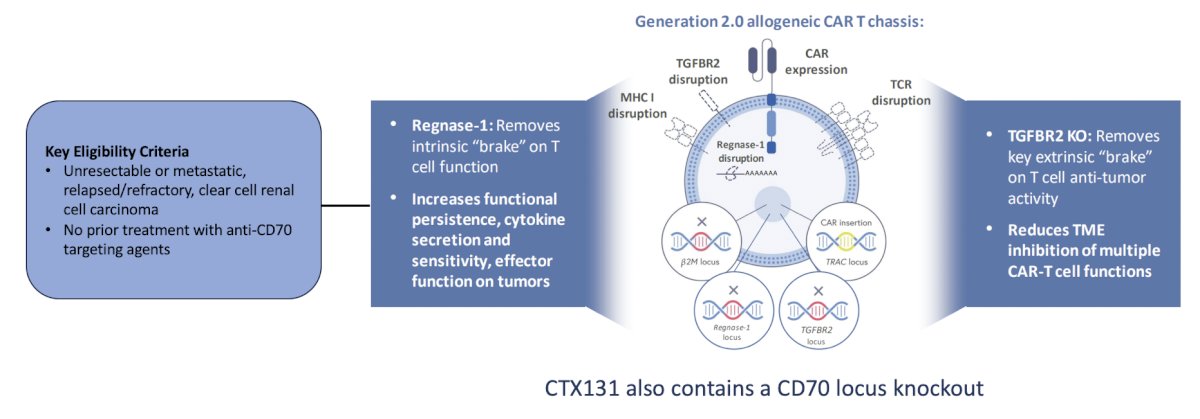
The second trial discussed by the panel was a phase 1, open label, dose escalation, and dose expansion study to investigate the safety, tolerability, and pharmacokinetic profile of AB521 monotherapy in patients with clear cell renal cell carcinoma and other solid tumors. The trial design for this trial, which is accruing at UCLA, is as follows: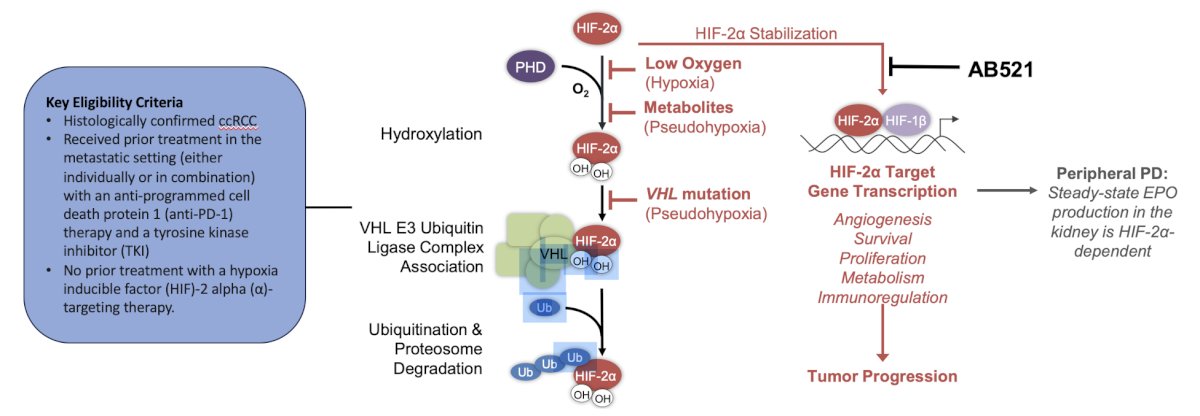
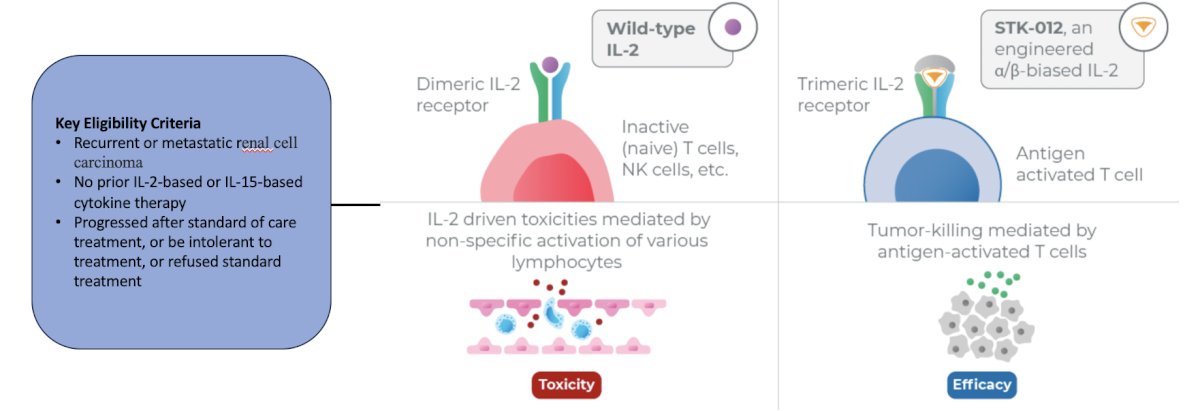
The third trial discussed by the panel in this disease space was a phase Ia/Ib study to evaluate the safety and tolerability of STK-012 monotherapy and in combination with pembrolizumab in patients with selected advanced solid tumors. The trial design for this trial, which is accruing patients at UC San Diego, is as follows:
The fourth trial discussed by the panel in the post first-line RCC was a phase 1, open label, multicenter study of BMS-986360/CC-90001 alone and in combination with chemotherapy or nivolumab in advanced solid tumors. The mechanism of action behind the hypothesis for this trial (accruing at Cedars Sinai Medical Center) is as follows: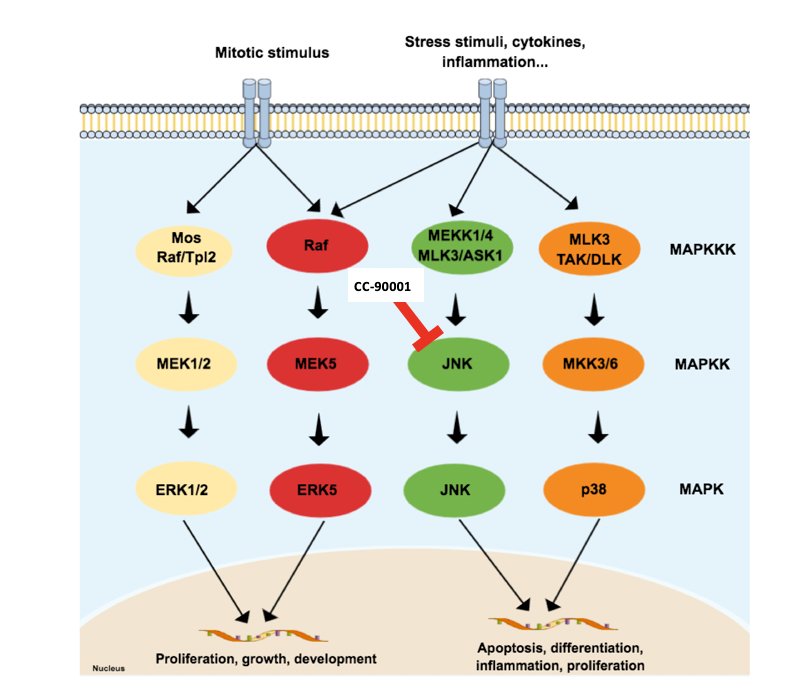
The fifth trial discussed by the panel was the RadiCal trial, a phase II randomized trial of radium-223 dichloride and cabozantinib in patients with advanced renal cell carcinoma with bone metastasis. Patients in this trial will be randomized 1:1 to cabozantinib + radium-223 (x 6 doses) versus cabozantinib alone. The primary outcome is SSE-free survival and secondary outcomes include safety, PFS, overall survival, and quality of life measures. This trial, accruing at UC San Diego, has the following trial design: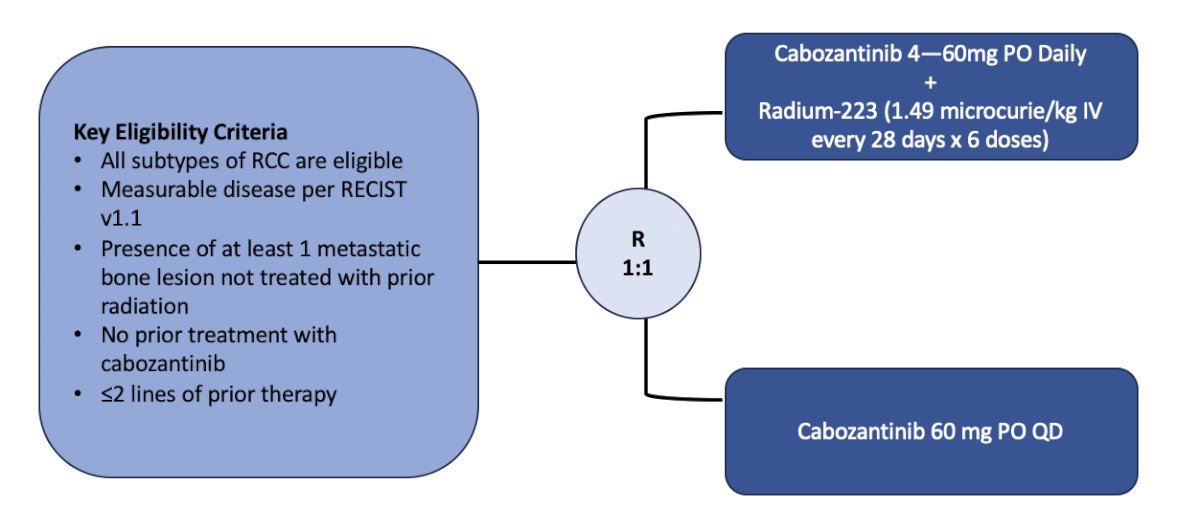
The sixth trial in this disease space discussed by the panel was a phase 1/2 study of IMC-F106C in advanced PRAME-positive cancer. The mechanism of action for the hypothesis of this trial is as follows: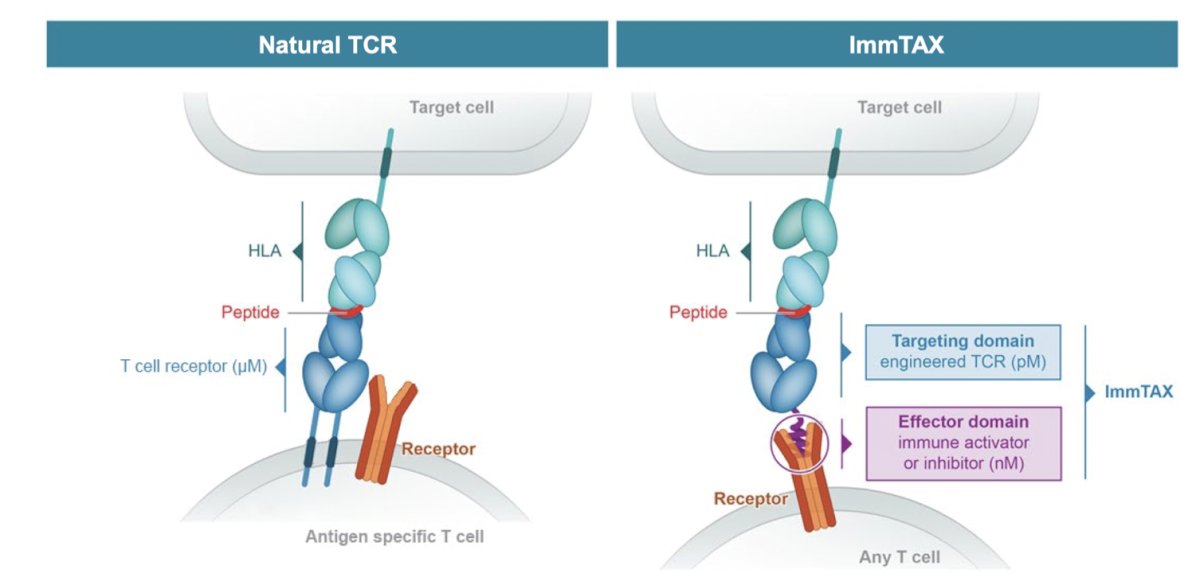
This trial is currently accruing at Cedars Sinai Medical Center.
The next trial discussed by the panel in the post first-line disease space is a phase 1b/2 dose-finding and expansion study evaluating the safety and efficacy of zanzalintinib (XL092) combined with either AB521 or AB521 + nivolumab in subjects with advanced clear cell renal cell carcinoma or other advanced solid tumors. The trial design for this trial (accruing at USC) is as follows: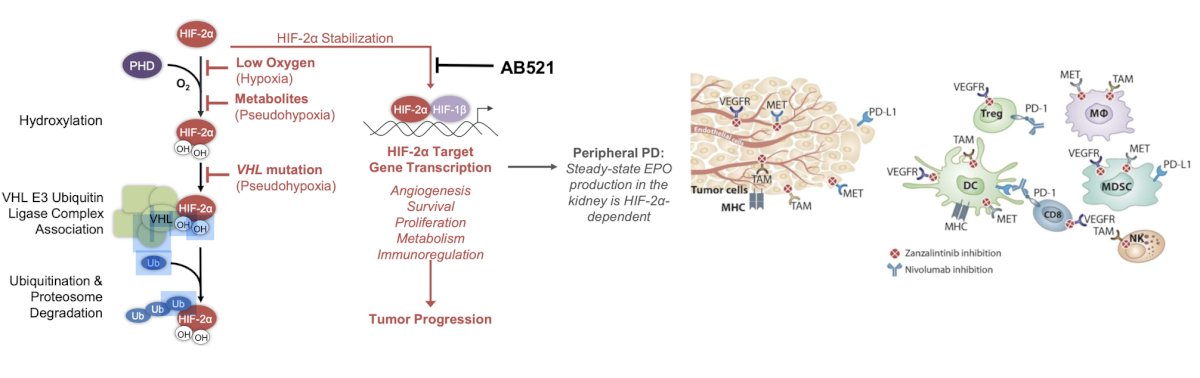
The final trial discussed by the panel in the post first-line disease space is a phase 1b/2 trial (accruing at Cedars Sinai Medical Center), multicenter, open label study, of S-531011 as monotherapy and in combination with an immune checkpoint inhibitor in participants with locally advanced or metastatic solid tumors. The mechanism of action for the hypothesis of this trial is as follows:
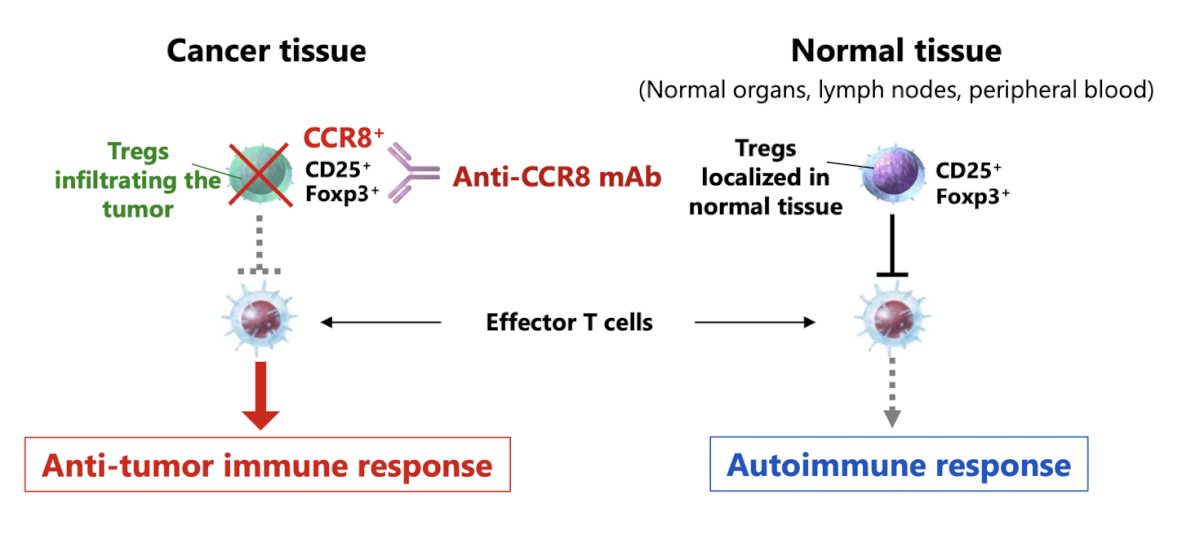
Next, the panel discussed non clear cell kidney cancer trials accruing patients in Southern California. The following table emphasizes trials in this disease space: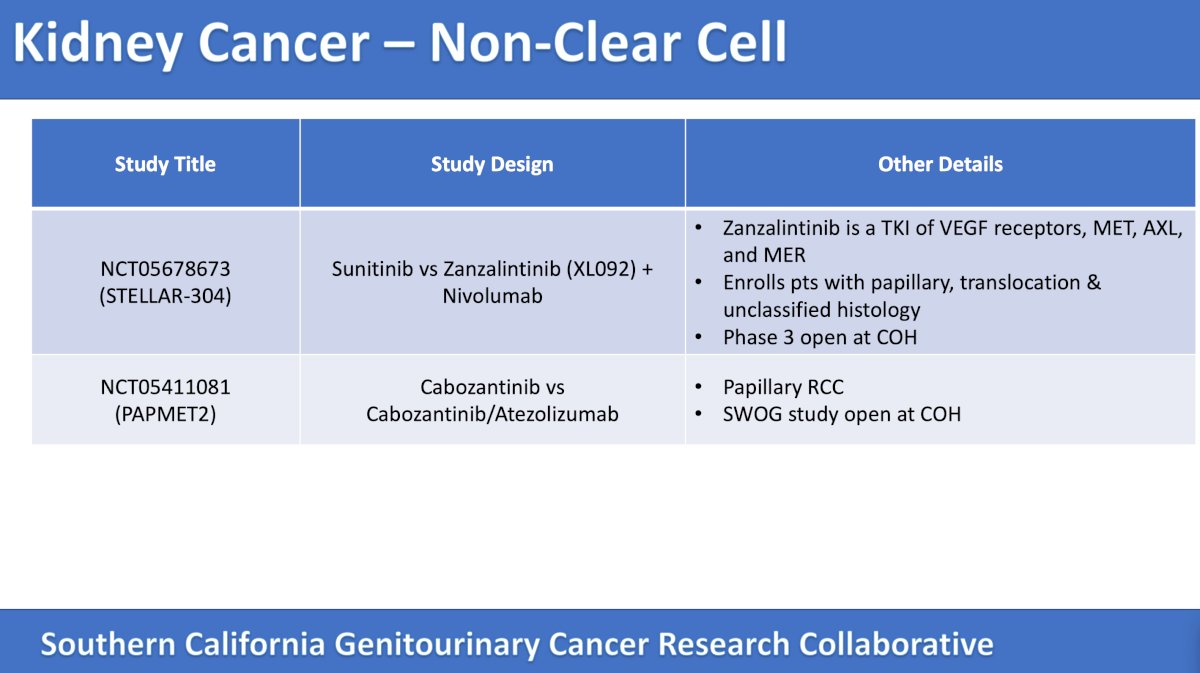
The panel then discussed the randomized phase 3 study of XL092 (zanzalintinib – a TKI of VEGFR2, MET, AXL, and MER) + nivolumab versus sunitinib in subjects with advanced or metastatic non-clear cell renal cell carcinoma. The primary outcome for this trial is PFS and objective response rate per RECIST, and the secondary outcomes are overall survival and safety. The trial design for this study accruing patients at City of Hope is as follows: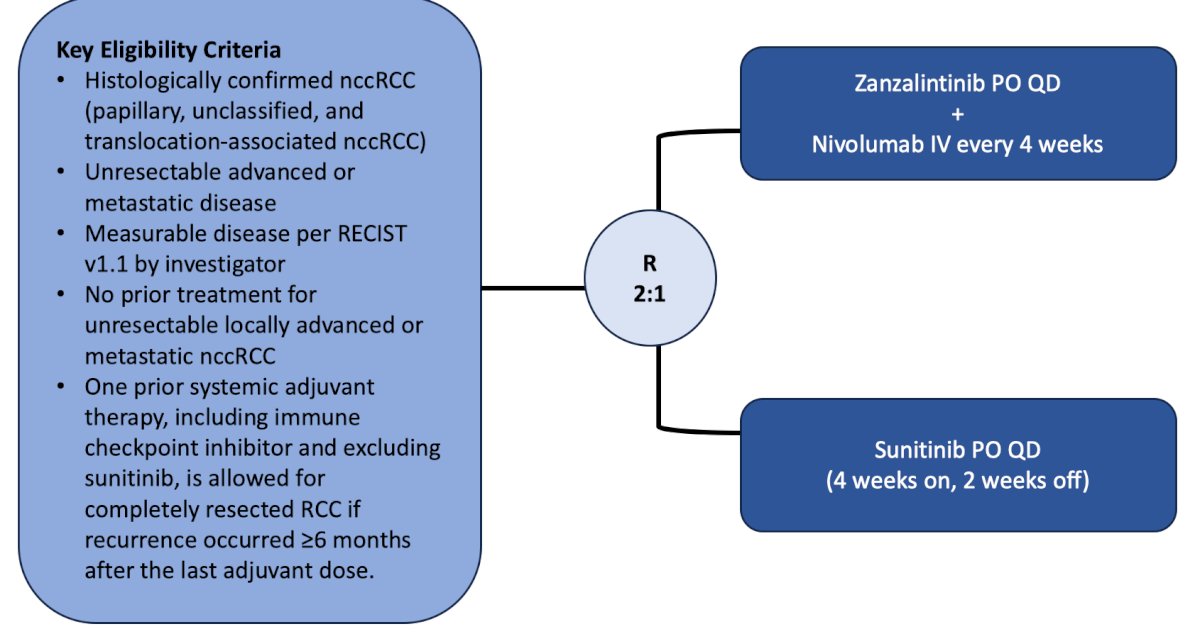
The second trial, accruing at City of Hope, is the PAPMET2 trial, a phase II randomized trial of cabozantinib with or without atezolizumab in patients with advanced papillary renal cell carcinoma. The primary outcome for this trial is PFS, and secondary outcomes include overall survival, objective response rate, and safety. The trial design for this trial is as follows: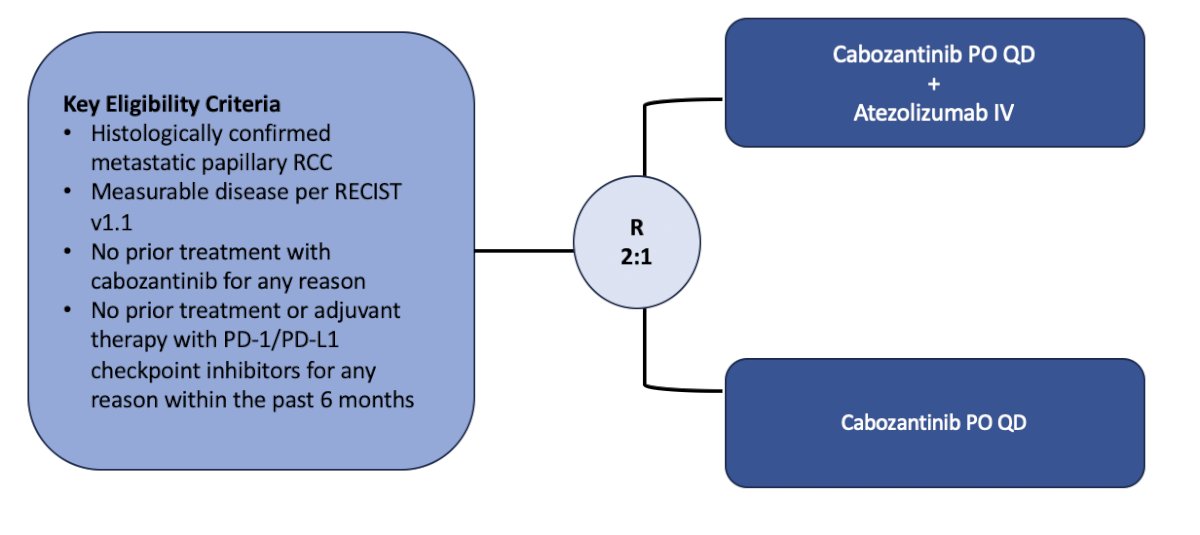
Finally, the panel concluded by discussing a diagnostic study from UCLA assessing 89Zr-TLX250 PET/CT versus contrast-enhanced CT for detection of recurrent clear cell renal cell carcinoma after surgery. The participant timeline for this trial is as follows: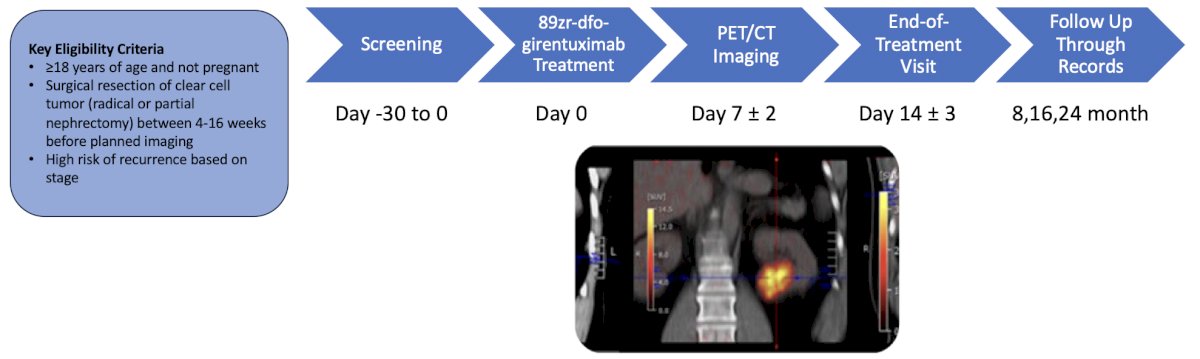
Moderated by: Monty Pal, MD, FASCO, City of Hope, Duarte, CA
Presented by:
- Rana R. McKay, MD, University of California San Diego, San Diego, CA
- Nataliya Mar, MD, University of California Irvine, Irvine, CA
- Brian Shuch, MD, University of California Los Angeles, Los Angeles, CA
- Wesley Yip, MD, City of Hope, Duarte, CA
- Jun Gong, MD, Cedars Sinai Medical Center, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Southern California Genitourinary Cancer Research Forum, Costa Mesa, CA, Fri, Mar 1, 2024.


