The 2024 Southern California Genitourinary Cancer Research Forum featured a prostate cancer session and a panel discussion of prostate cancer studies for localized, salvage radiotherapy/BCR, mHSPC, and mCRPC. Moderator Dr. Rana McKay started by highlighting the available trials in Southern California for patients with localized prostate cancer:

The first trial discussed in detail by the prostate cancer panel was the phase 2, single arm NePtune trial, accruing patients at UC San Diego. Patients with germline or somatic BRCA 1/2 alterations and localized prostate cancer will receive ADT + olaparib for 6 months followed by radical prostatectomy. The primary endpoint is pathologic complete response or minimum residual disease (tumor <= 5 mm), and secondary endpoints include PSA, surgical staging, surgical margin rate, safety, and time to testosterone recovery. The trial design for NePtune is as follows:
The second trial in the localized disease space discussed was the phase 2b INTREPId multicenter trial, accruing patients at UC San Diego. Patients are being randomized to bicalutamide + GnRH agonist + radiation therapy (starting 4-16 weeks after ADT) versus darolutamide + radiation therapy (starting 4-16 weeks after darolutamide). The primary outcome is the percentage of patients with a PSA nadir <= 0.5, and secondary outcomes include a percentage of patients with good erectile function at 3 months from the end of treatment, PSA progression free survival, and metastasis free survival. The trial design for INTREPId is as follows:
The third trial discussed in detail by the panel was the UCLA HEATWAVE phase 2, single arm trial. Among NCCN unfavorable intermediate risk prostate cancer, patients will receive apalutamide + SBRT for 5 fractions over 1-2 weeks with a primary endpoint of the percentage of patients achieving a PSA < 0.2 ng/mL. Secondary endpoints include time to biochemical recurrence, patient reported outcomes, radiographic persistence of disease on PSMA PET and MRI, as well as acute and late toxicities. The trial design for HEATWAVE is as follows:
The next trial discussed in the localized prostate cancer space was the Cedars Sinai Medical Center NADIR phase 2 randomized clinical trial. Patients with high risk localized prostate cancer will be treated with ADT + niraparib for 2 months followed by dose-escalated IMRT and then randomized to niraparib for 10 months + ADT for 22 months versus both niraparib + ADT for 22 months. The primary endpoint is maintenance disease free survival, and secondary endpoints include overall survival, prostate cancer specific survival, pathologic complete response, time to distant metastasis, adverse events, and time to local/regional or distant progression. The trial design for NADIR is as follows: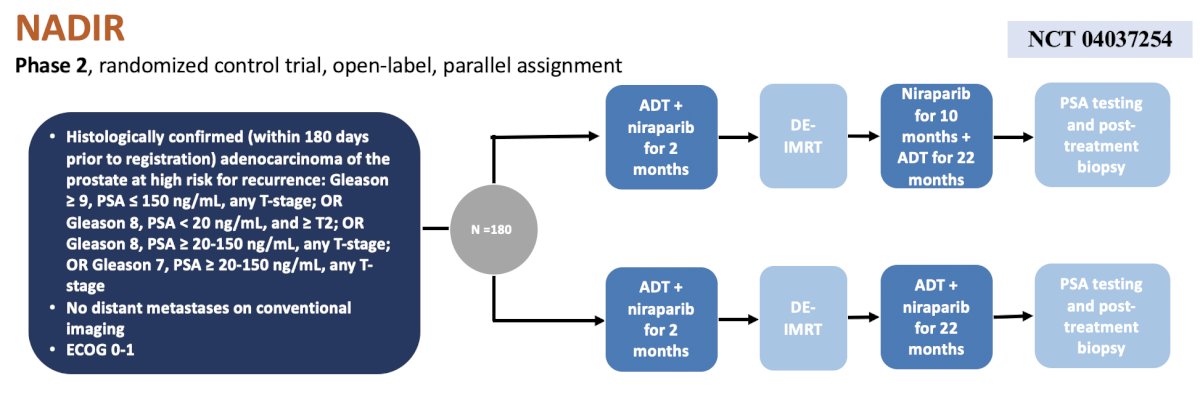
The fifth trial in the localized space was a phase 2a, single arm, open label trial at USC assessing low dose daily apalutamide prior to radical prostatectomy. The primary endpoint is change in PSA levels and secondary endpoints include reversibility of testosterone levels, plasma apalutamide concentrations, and health related quality of life: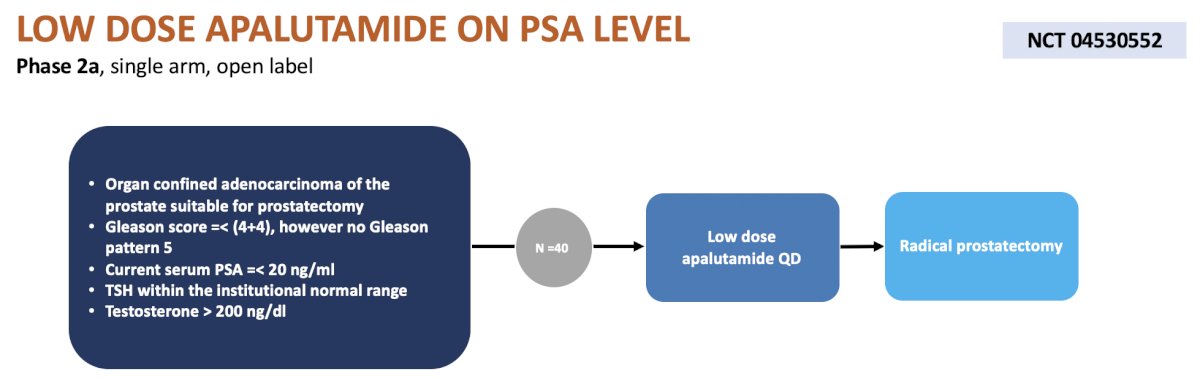
The following table highlights the two trials in Southern California for patients with N1 prostate cancer:
A phase 1 trial at UCLA is assessing biodistribution of 99mTc-PSMA-I&S among men with N1 prostate cancer. After undergoing 99mTc-PSMA-I&S, patients will undergo a radical prostatectomy + pelvic lymph node dissection. The primary endpoint is biodistribution of 99mTc-PSMA-I&S in normal and malignant tissues, and secondary endpoints include 99mTc-PSMA-I&S accumulation within tumor lesions observed by in vivo SPECT correlated with PSMA expression and best time point for 99mTc-PSMA-I&S radioguided surgery: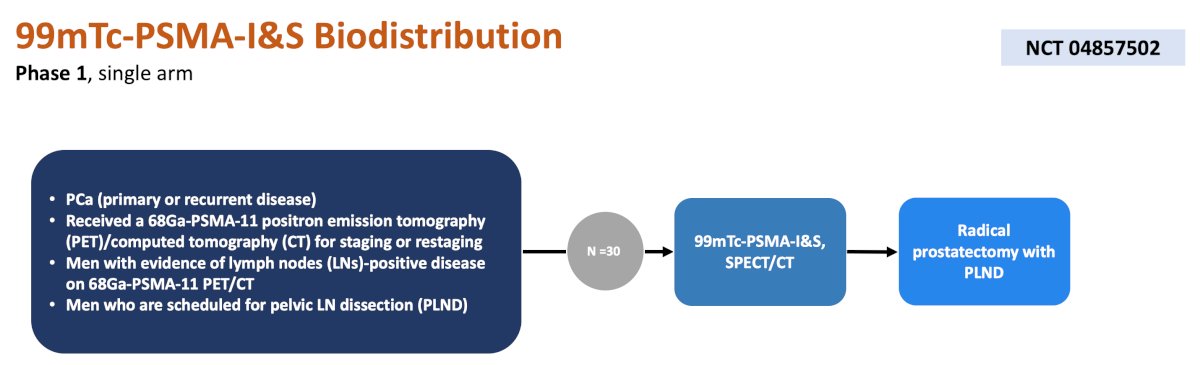
The second trial is the NRG-GU008 INNOVATE phase 3 trial at USC which is randomizing pN+ patients with a detectable PSA after radical prostatectomy to ADT for 24 months + EBRT for 7 weeks versus ADT + apalutamide + abiraterone/prednisone for 24 months + EBRT. The primary endpoint is metastasis free survival, and secondary endpoints include quality of life, overall survival, biochemical progression free survival, time to locoregional progression, and adverse events:
In the biochemical recurrent prostate cancer disease space, there is a phase I open label trial at City of Hope randomizing 36 patients 2:1 to white button mushroom extract (Agaricus bisporus) orally daily for 48 weeks versus observation. The primary endpoint for this trial is PSA levels from baseline to 12 weeks of follow-up:
In the mHSPC disease space, there are several exciting clinical trials accruing patients in Southern California: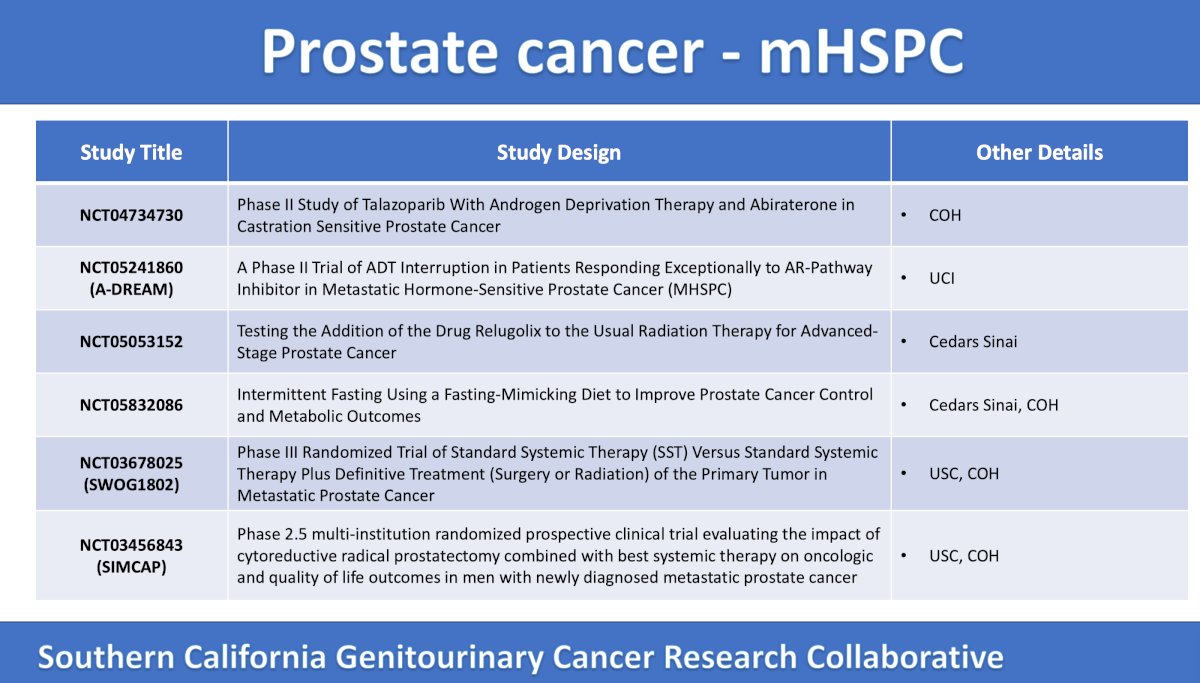
The first trial discussed by the panel for mHSPC was a phase 2, single arm, open label trial at City of Hope treating 70 patients with ADT + abiraterone + prednisone + talazoparib daily. The primary endpoint for this trial is PSA nadir < 0.2 at 12 months, and secondary endpoints include objective response rate, PSA response, radiographic progression free survival, and patient reported outcomes: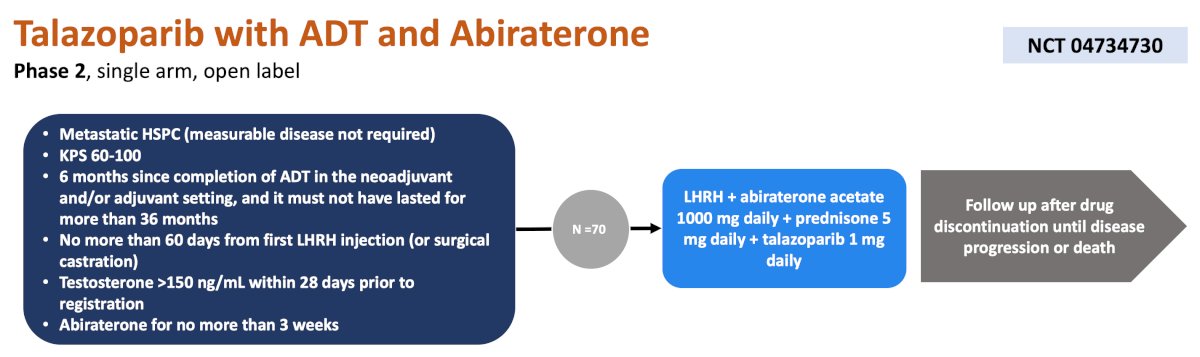
The second trial for mHSPC is the UC Irvine A-DREAM phase 2, single arm trial assessing the utility of an ARSI and interrupted ADT among 75 patients. The primary endpoint is treatment free at 18 months (with a normal testosterone), and secondary outcomes include radiographic progression free survival, time to next treatment, overall survival, and cost. The trial design for A-DREAM is as follows: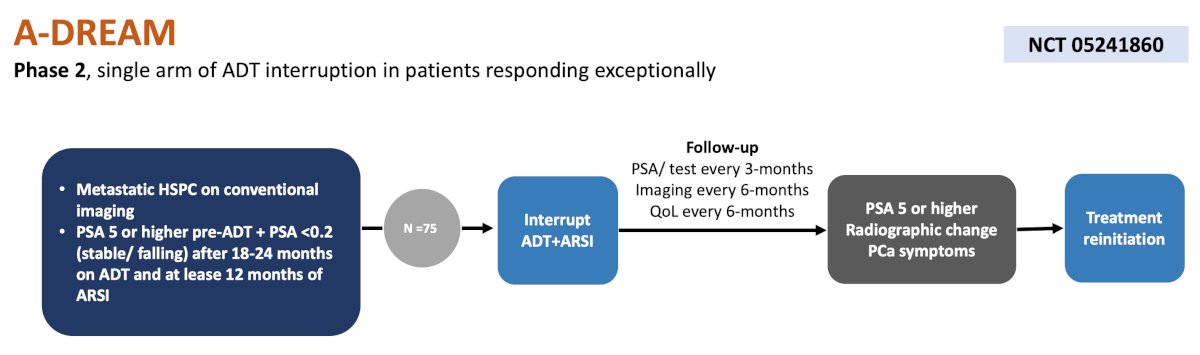
The next trial discussed by the panel was the phase 2 NRG PROMETHEAN trial accruing patients at Cedars Sinai Medical Center. This trial is randomizing 260 patients with 1-5 oligometastatic lesions to placebo + SBRT for 1-3 weeks versus relugolix + SBRT for 1-3 weeks. The primary outcome is radiographic progression free survival, and secondary endpoints include PET-progression free survival, metastasis free survival, overall survival, sexual function, and fatigue. The trial design for NRG PROMETHEAN is highlighted below:
The fourth trial discussed is a City of Hope and Cedars Sinai Medical Center phase 2, multi site study randomizing men 1:1 to intermittent fasting using a fasting mimicking diet versus a standard anti-cancer diet. The primary endpoint is response to cancer treatment, and secondary endpoints include time to development of castration resistance and metabolic toxicity: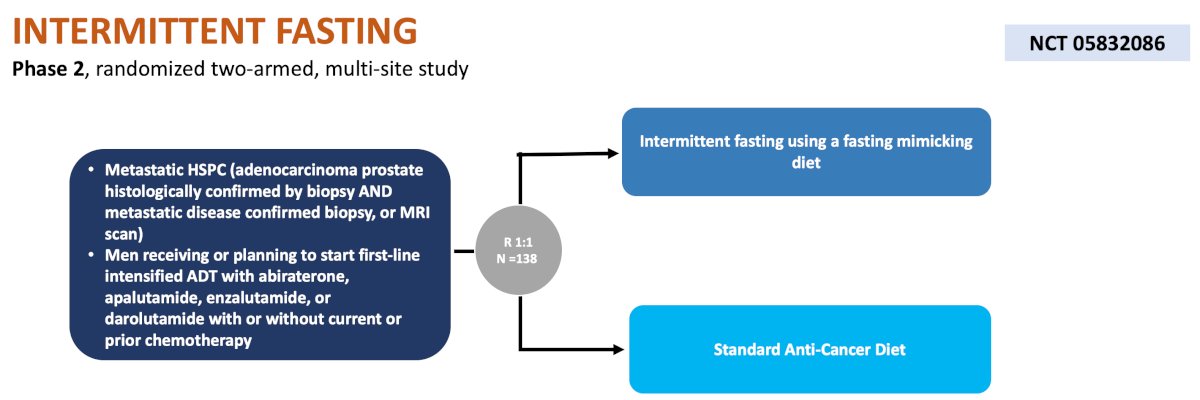
The phase 3 SWOG 1802 trial is accruing patients at City of Hope and USC, which is randomizing mHSPC patients >= M1a to systemic standard of care + definitive treatment (surgery or radiation) versus systemic standard of care. The primary endpoint is overall survival, and secondary endpoints include rate of symptomatic local progression and progression free survival. The trial design for SWOG 1802 is as follows:
The final trial discussed in the mHSPC space was the SIMCAP phase 2.5, multi-institutional, randomized trial accruing patients at City of Hope and USC. This trial is similar to SWOG 1802 and is randomizing patients to best systemic therapy versus best systemic therapy followed by receipt of cytoreductive prostatectomy + extended pelvic lymph node dissection one month later. The primary endpoint is failure free survival at 2 years (biochemical recurrence, progression, or death), and secondary endpoints include time to biochemical progression, cancer-specific survival, and complication rates: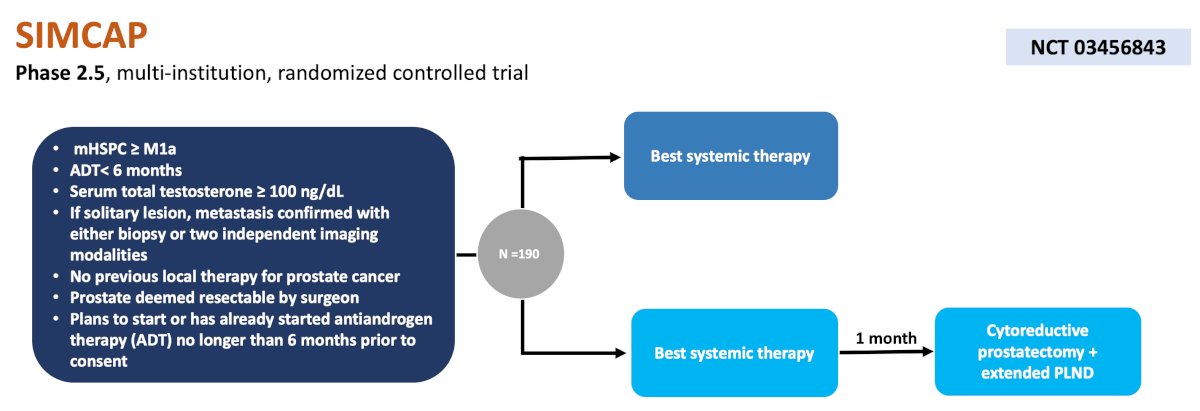
Dr. McKay highlighted that there are 11 mCRPC trials in Southern California enrolling patients: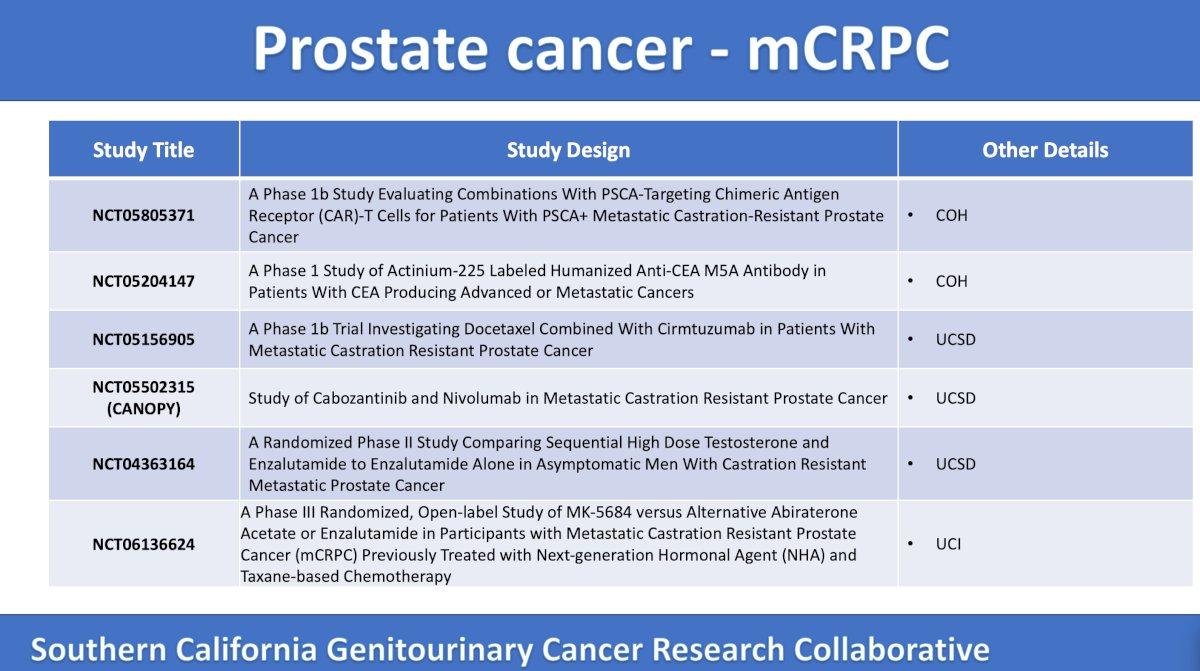
The first trial discussed is a phase 1b trial at City of Hope assessing combinations with PSCA-targeting CAR-T cells for PSCA+ tumors. The primary endpoints are adverse events and PSA50 response, and secondary endpoints are overall survival, progression free survival, and disease response:
The second trial is a phase 1b trial at City of Hope assessing actinium Ac225 anti-CEA mAb in patients with neuroendocrine differentiated prostate cancer with a histologic assessment of CEA expression. These 20 patients will be treated with Ac225-DOTA-M5A anti-CEA antibody, with a primary endpoint of maximal tolerated dose. Secondary endpoints include clinical activity of the agent in metastatic CEA expression cancer, organ biodistribution, and pharmacokinetics: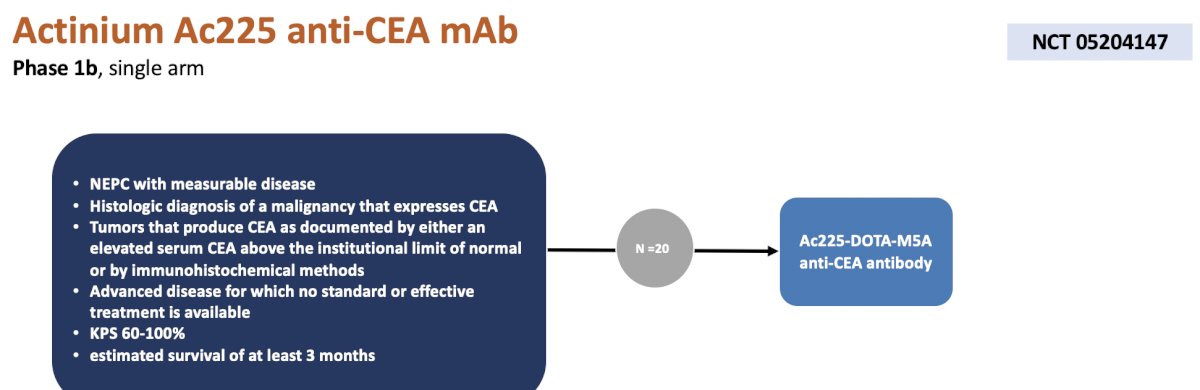
The third trial discussed by the panel is a UC San Diego phase 1b, single arm trial assessing docetaxel in combination with cirmtuzumab (mechanism of action: mAb anti-ROR1). Among 32 patients, they will receive induction docetaxel + cirmtuzumab on days 1, 15, and 29 followed by maintenance docetaxel + cirmtuzumab every 21 days. The primary endpoint is recommended phase 2 dose, and secondary endpoints include adverse events, alkaline phosphatase response, time to PSA progression, radiographic progression free survival, overall survival, and time to first skeletal related event:
Next, the panel discussed CANOPY (HCRN GU21-517), a phase 2, two-stage trial accruing at UC San Diego whereby 50 patients will undergo cabozantinib + nivolumab followed by an on treatment biopsy followed by cabozantinib + nivolumab. The primary endpoint is radiographic progression free survival, and secondary endpoints include objective response rate, 6-month radiographic progression free survival, 6-month PSA response, overall survival, and time to PSA progression. The trial design of CANOPY is as follows:
The STEP-UP trial is a phase 2, three arm trial accruing at UC San Diego whereby men are randomized to enzalutamide versus enzalutamide alternating with BAT versus BAT until PSA progression followed by enzalutamide. The primary endpoint is clinical progression free survival or radiographic progression free survival, and secondary endpoints include safety, PSA response rate, quality of life, objective response rate, and overall survival. The trial design for STEP-UP is as follows:
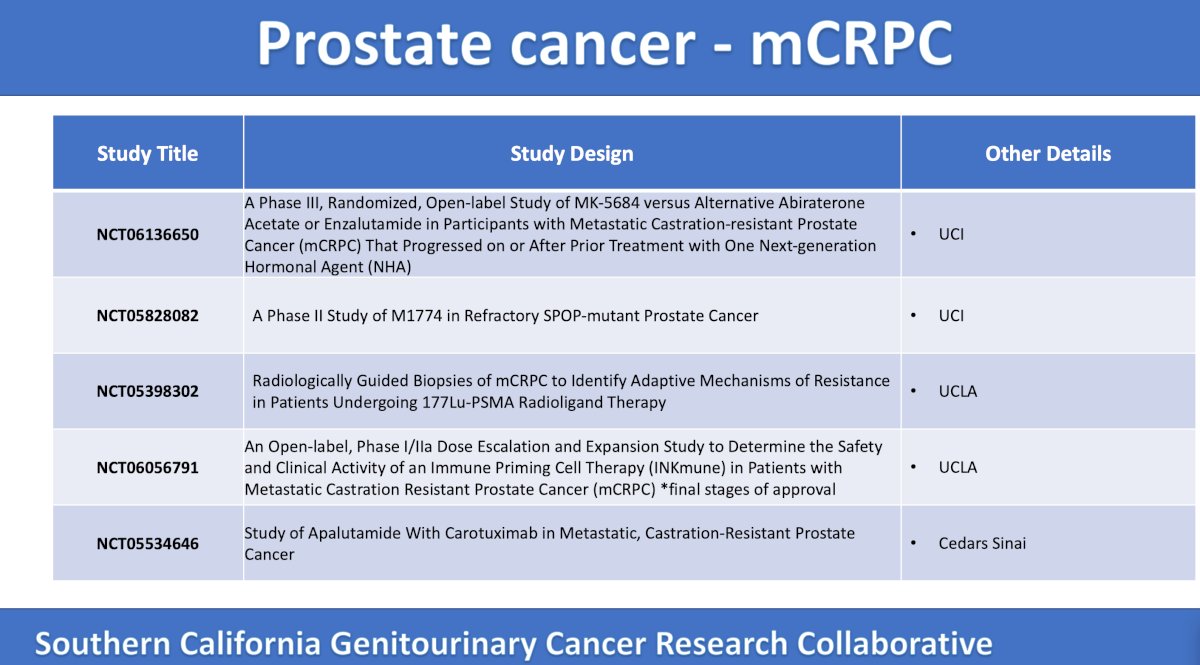
Next, the panel discussed a phase 3 trial accruing at UC Irvine assessing MK-5684 (mechanism of action: oral, non-steroidal, selective inhibitor of CYP11A1) versus alternative novel hormonal antiandrogen among mCRPC patients with a prior taxane. The primary endpoints are overall survival, overall survival in AR LBD mutations, and radiographic progression free survival. Secondary outcomes include time to initiation of the first subsequent anticancer therapy, objective response rate, duration of response, time to pain progression, PSA response rate, adverse events, and time to first symptomatic skeletal related event:
The 7th trial in the mCRPC disease space discussed by the panel is a phase 1b single arm trial of M1774 in refractory SPOP-mutant prostate cancer. In this trial, 20 patients will be treated with tuvusertib (mechanism of action: elective and orally active ATR inhibitor) daily, with a primary endpoint of objective response rate and secondary endpoints of overall survival, progression free survival, adverse events, and SPOP-driven signature changes: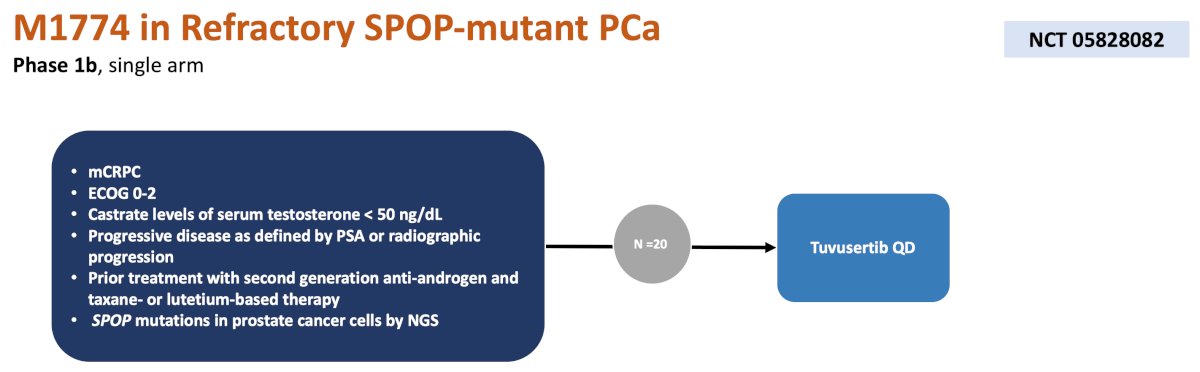
The next trial is a UCLA phase 1, single arm trial to assess guided biopsies to identify mechanisms of resistance. There will be 30 patients that will undergo baseline image-guided biopsy followed by Lu-PSMA and follow-up image guided biopsy. The primary endpoint is the proportion of mCRPC patients with molecular and cellular alterations in the tumor, immune, and stromal cells after radioligand therapy:
Next, the panel discussed the UCLA phase 1/2a open label, dose escalation, and expansion CaRe prostate trial with the following design:
The mechanism of action of INKmune is a biologic delivery system and method for cancer treatment, in vivo priming, and activation of natural killer cells. The primary endpoint is the optimal concentration of INKmune therapy to be used in patients with mCRPC and safety.
The final trial discussed by the panel is a phase 2 randomized trial accruing at Cedars Sinai Medical Center whereby 90 men will be randomized to apalutamide daily versus apalutamide daily + carotuximab (mechanism of action: mAb potently inhibiting CD105-mediated signaling). The primary endpoint is radiographic progression free survival, and secondary endpoints include adverse events, objective response rate, and biochemical progression free survival:
Moderated by: Rana R. McKay, MD, University of California San Diego, San Diego, CA
Presented by:
- Tanya Dorff, MD, City of Hope, Duarte, CA
- Amar U. Kishan, MD, University of California Los Angeles, Los Angeles, CA
- Arash R. Kalebasty, MD, University of Irvine, Irvine, CA
- Jun Gong, MD, Cedars Sinai Medical Center, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Southern California Genitourinary Cancer Research Forum, Costa Mesa, CA, Fri, Mar 1, 2024.


