To this end, the Johns Hopkins Greenberg Bladder Cancer Institute and the American Urological Association held a Translational Research Collaboration entitled “Drug Development in NMIBC from Scientific, Regulatory, Clinician, and Patient Perspectives”. The fourth session of this symposium focused on the Treatment of Non-Muscle-Invasive Urothelial Cancer of the Upper Tract. In this session, Dr. Margulis presented on the role of perioperative chemotherapy and targeted therapy in locally advanced upper tract urothelial carcinoma (UTUC).
To begin, Dr. Margulis highlighted problems with the current treatment paradigm in patients with UTUC, highlighting issues of overtreatment of patients with small low-grade tumors with nephroureterectomy as well as the undertreatment of those with high-grade invasive tumors who harbor a significant risk of occult metastasis.
Particularly among patients with these more aggressive phenotypes, there is a rationale for perioperative chemotherapy, in part based on the fact that death results from distant disease, which is unable to be treated by local interventions. Further, there are proven benefits to chemotherapy in urothelial cancer of the bladder. Peri-operative chemotherapy may be administered either with adjuvant or neoadjuvant intent, with various benefits to each approach.
In the context of adjuvant chemotherapy, Dr. Margulis highlighted five potential rationales:
1. Where the local tumor is the primary problem (eg. due to symptomatic disease), this will be immediately surgically managed.
2. With surgery performed upfront, the decision for the provision of chemotherapy can be based on true, final pathology.
3. Relatedly, pre-operative staging of UTUC is difficult. As a result, routine use of neoadjuvant chemotherapy may result in overtreatment.
4. An upfront surgical approach prevents compromise of local therapy due to toxicity.
5. There is no delay in definitive local therapy, in particular for those in whom chemotherapy may be ineffective.
Supporting this adjuvant approach, the phase III POUT trial provided level 1 data. This trial randomized patients with invasive UTUC following nephroureterectomy to undergo either surveillance or platinum-based chemotherapy, with stratification to gemcitabine plus either cisplatin or carboplatin according to baseline renal function.
As previously reported and published, Dr. Margulis highlighted that the POUT trial met its primary endpoint demonstrating improved disease-free survival among patients who received adjuvant chemotherapy (71% vs 0.54% at 2-years; hazard ratio 0.49, 95% CI 0.31-0.76). This trial further demonstrated benefits in secondary outcomes including metastasis-free survival (74% vs 60% at 2-years; hazard ratio 0.49, 95% CI 0.30-0.78) though overall survival are to date immature. However, he emphasized that adjuvant chemotherapy is associated with non-trivial rates of treatment-related toxicity (grade 3 or greater: 53% vs 14%). In spite of this, most people randomized to adjuvant therapy were able to receive therapy.
Dr. Margulis then highlighted subgroup analyses suggesting that patients most likely to benefit from adjuvant chemotherapy are those without nodal involvement, who are able to receive gemcitabine and cisplatin, and those with negative margins.
In the context of bladder cancer, previous work in patients with high risk of disease recurrence and progression, there is evidence of a benefit to early (compared to delayed) administration of chemotherapy prior to the development of the micrometastatic disease.
Dr. Margulis then highlighted a potential rationale for a neoadjuvant approach:
1. This gives the opportunity to administer systemic therapy when the blood and lymphatic supply is intact.
2. Prior to surgery, patients are more likely to be able to tolerate chemotherapy.
3. Using consolidative surgery, a neoadjuvant approach to chemotherapy allows an in vivo chemosensitivity test when assessing pathologic complete response rates.
4. By giving systemic therapy first, this treats micrometastatic disease immediately.
5. Further, surgery may be facilitated by decreasing tumor mass.
6. Additionally, when upfront surgery may lead to complications which delay or prevent adjuvant therapy, a neoadjuvant approach ensure the receipt of systemic therapy.
7. Finally, patients commonly refuse adjuvant therapy after surgery so a neoadjuvant approach may improve use of systemic therapy.
Among these, the strongest rationale for neoadjuvant use is that many will not be candidates for platinum following nephroureterectomy. Dr. Margulis highlighted that approximately half of patients have eGFR < 50 mL/min/1.73 m2 prior to surgery with this number rising to 75% or more following surgery.
While level 1 data are lacking, observational studies, including for example from MD Anderson, demonstrate a potential benefit to neoadjuvant treatment with significant rates of down staging and a complete pathologic response in approximately 15%.
The prospective EA8141 trial led by Dr. Margulis provided non-randomized data supporting neoadjuvant MVAC, which was associated with a 14% complete response rate and 62% downstaging rate to superficial (pT1 or less) disease.
However, this treatment is not without toxicity, though approximately 80% of patients are able to complete trial per protocol criteria. Other trials have demonstrated similar benefits to the use of neoadjuvant chemotherapy though none provide a randomized comparison with observation or and adjuvant approach.
Dr. Margulis highlighted a number of ongoing neoadjuvant chemotherapy trials including comparisons of neoadjuvant cisplatin and gemcitabine followed by nephroureterectomy versus nephroureterectomy alone (NCT02876861), of neoadjuvant gemcitabine, cisplatin, and the PD-1 inhibitor toripalimab) in both UTUC and muscle invasive bladder cancer, as well as a feasibility comparison of neoadjuvant and adjuvant chemotherapy in UTUC (URANUS) with the endpoint being the feasibility to deliver all planned cycles of chemotherapy.
He further highlighted the rationale for immunotherapy in UTUC, emphasizing that mismatch repair-deficient tumors are more responsive to PD-1 blockade. In the KEYNOTE-045 trial of second line pembrolizumab in metastatic urothelial cancer, a greater benefit to treatment was seen in UTUC than in bladder cancer. A number of ongoing phase III adjuvant immunotherapy studies are designed to accrue patients with urothelial cancer, allowing those with UTUC.
Additionally, in parallel to the completed EA8141 prospective non-randomized assessment of neoadjuvant chemotherapy, Dr. Margulis described the EA8192 trial which will provide randomized data (among patients with adequate renal function) regarding the benefit of combined chemotherapy with immune checkpoint inhibition versus chemotherapy alone.
In addition to immune checkpoint inhibition, there is the potential for targeted therapy: FGFR aberrations are found most commonly in UTUC, compared to all other tumor types. Thus, there is demonstrated proof-of-concept for the targeting of FGFR3 in patients with mutant metastatic UTUC using pan-FGFR inhibitors. The ongoing PROOF 302 trial will test this approach in a randomized comparison of adjuvant infigratinib versus placebo in patients with invasive urothelial cancer with susceptible FGFR3 alterations.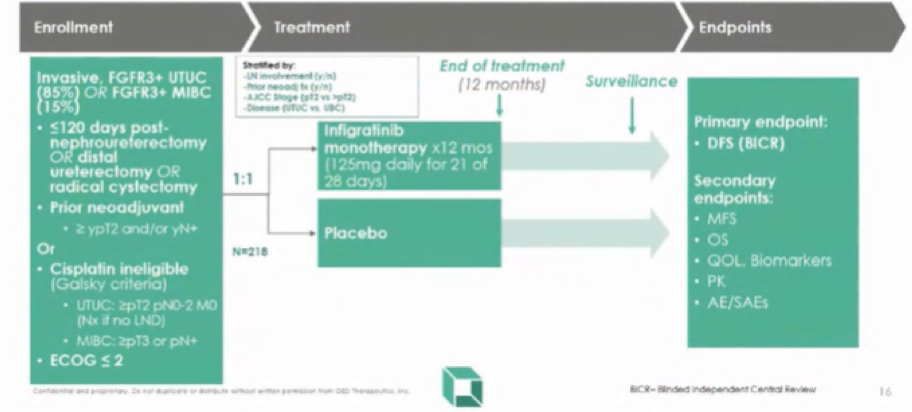
A similar approach is being tested by Dr. Matin in a phase 1b design (NCT04228042).
In conclusion, Dr. Margulis considered ways in which we may improve the effect of perioperative chemotherapy. First, it is important to refine staging to identify high-risk individuals who are likely to progress following surgery alone. This will allow us to minimize morbidity and cost in those who don’t need chemotherapy. Second, we need to better identify patients who are likely to respond to neoadjuvant chemotherapy so that we can improve response rates and stimulate the development of new therapies for those who are unlikely to respond to our current treatments. In the bladder cancer space, work with comprehensive molecular characterization offers promise: in UTUC, most patients have a luminal papillary phenotype.
Presenter: Vitaly Margulis, MD, Associate Professor of Urology at UT Southwestern Medical Center


