Dr. Kates notes that clinicopathologic parameters are currently the best predictors of response to BCG. Historical data from previous EORTC trials (n=2,595) suggests that a simple scoring system using the number of tumors, tumor size, prior recurrence rate, T stage, carcinoma in situ, and grade is able to predict probabilities of disease recurrence and progression.1 For example, the time to the first recurrence stratified by recurrence score is as follows:

Dr. Kates states that the mechanism of action of BCG non-specific immunotherapy in NMIBC is very complex. BCG attached to the urothelium (via fibronectin) is internalized by bladder cancer cells causing a direct cytotoxicity of immune system effectors (upregulation in expression of MHC-II and ICAM-I) to kill bladder cancers (activated immune cells such as CD4 and CD8 T cells).2 Various immune cells infiltrate bladder tumor tissues, releasing large amounts of inflammatory cytokines and chemokines, thus changing the microenvironment:

The immune checkpoint and BCG response has been evaluated, notably nearly 15 years ago by Brant Inman and colleagues, specifically assessing the role of PD-L1 as a mechanism for local stage progression.3 They found that PD-L1 expression was observed in 7% of pTa, 16% of pT1, 23% of pT2, 30% of pT3/4, and 45% of carcinoma in situ tumors. Furthermore, PD-L1 expression was associated with high-grade tumors (OR 2.4, p = 0.009) and tumor infiltration by mononuclear cells (OR 5.5, p = 0.004).
Dr. Kates and his team have built off of this initial work of immune checkpoint and BCG response looking at adaptive immune resistance to intravesical BCG, with a recent publication in Clinical Cancer Research.4 Among 31 patients that were BCG responders and 32 BCG non-responders, they compared immune cell populations between BCG responders and non-responders, and between pretreatment and post-treatment tumor samples. They found no differences in CD4, CD8, or FoxP3 expression identified between BCG responders and non-responders. Additionally, baseline PD-L1 expression (22C3 and SP-142) was observed in 25% to 28% of non-responders and 0% to 4% of responders (p < 0.01), with PD-L1+ cells in BCG non-responders co-localized with CD8+ T cells; BCG therapy did not increase PD-L1 gene expression (RNA-seq) or protein levels. Furthermore, they found that the number of pretreatment CD4+ T cells was very low among PD-L1+ non-responders (12%) and high among PD-L1- non-responders (50%, p < 0.01).
Taken together, immune phenotypes have been developed ranging from inflamed (IFN-gamma signal) to immune excluded (TGF-beta signal) to non-inflamed (immune desert – fatty acid metabolism):

Dr. Kates notes that there are also genomic predictors of BCG response, including recent work from Dr. Joaquim Bellmunt and his group.5 This study assessed exome sequencing on 62 high-grade T1 and 15 matched normal tissue samples, studying somatic mutations, copy-number alterations, mutation load, and mutation signatures. DNA damage response gene mutations were associated with higher tumor mutational burden (p < 0.0001) and good outcomes (p = 0.003). Additionally, mutations in ERCC2 and BRCA2, as well as APOBEC-A and ERCC2 mutant tumors (COSMIC5) were also associated with good outcomes (p = 0.047; p = 0.0002). Mutations associated with TP53, ATM, ARID1A, AHR, and SMARCB1 were more frequent in patients with disease progression, as were focal copy-number gain in CCNE1 and CDKN2A deletion (p = 0.047; p = 0.06).
Recent work by Robertson et al. [6] has led to the identification of differential tumor subtypes among patients with T1 bladder cancer. These authors used transcriptome profiling and unsupervised clustering to identify five consensus subtypes (T1-Myc, T1-LumGU, T1-Inflam, T1-TLum, T1-Early) of T1 tumors treated with repeat transurethral resection and induction and maintenance BCG, which is summarized in the following table: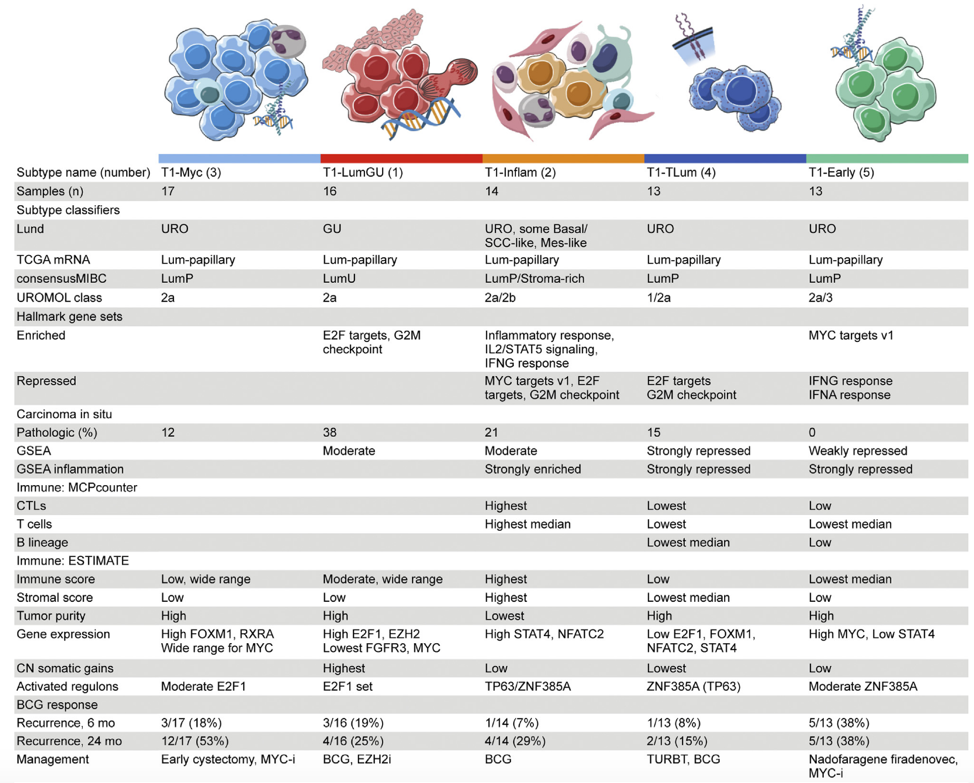
Work from Dr. Kates’ lab is also looking to assess the association between molecular subtypes and BCG response with the Hopkins BCG cohort currently consisting of 30 BCG responders and 54 BCG non-responders. Initial work shows an increase in p53 among BCG non-responders and activated TGF-beta signaling in the immune excluded subtype of non-responders.
Dr. Kates notes that there is an important caveat to predicting response to BCG, specifically delineating markers that are predictive of BCG response versus prognostic (ie. the patient’s cancer will recur or progress regardless of the therapy we choose). This is an important distinction as new therapies are FDA approved that seek to maintain bladder preservation. A randomized trial comparing BCG versus agent X for BCG naïve NMIBC is likely the best way to differentiate a predictive versus prognostic biomarker. The S1602 PRIME trial is a pathway to discovery, randomizing patients to intravesical TICE BCG versus intravesical Tokyo BCG versus prime (intradermal Tokyo strain BCG) plus intravesical Tokyo BCG, with an estimated enrolment >1,000.
Dr. Kates concluded his presentation with several important take-home points:
- The mechanism of BCG response is complex and therefore predictors of response will likely be multifactorial
- Tumor mutational burden, ERCC2, and ARID1A may attenuate BCG response and should be further explored and validated
- Activated T cells (CD4 and CD8) are necessary to overcome adaptive immune resistance mediated partially through PDL1 positivity, and activated T cell signatures are potential biomarkers of response
- Immune inflamed, immune excluded, and immune desert phenotypes may all be implicated in BCG resistance and predictive biomarkers of BCG response should target each of these pathways
- BCG non-responders show evidence of an immune excluded and immune desert phenotype, with the TGFbeta pathway activated in the immune excluded subtype
Presented by: Max Kates, MD, Director, Bladder Cancer Program, Assistant Professor of Urology, John Hopkins School of Medicine, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md during the 4th annual bladder cancer translational research meeting, co-sponsored by the American Urological Association (AUA) and the Johns Hopkins Greenberg Bladder Cancer Institute, March 4-6, 2021
References:
- Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combine analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006 Mar;49(3):466-475.
- Li J, Zhan L, Qin C. The double-sided effects of Mycobacterium Bovis bacillus Calmette-Guerine vaccine. NPJ Vaccines. 2021 Jan 25;6(1):14.
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: Associations with localized stage progression. Cancer2007 Apr 15;109(8):1499-1505.
- Kates M, Matoso A, Choi W, et al. Adaptive Immune Resistance to intravesical BCG in Non-muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin Cancer Res. 2020 Feb 15;26(4):882-891.
- Bellmunt J, Kim J, Reardon B, et al. Genomic Predictors of Good Outcome, Recurrence, or Progression in High-Grade T1 Non-Muscle-Invasive Bladder Cancer. Cancer Res.2020 Oct 15;80(20):4476-4486.
- Robertson AG, Groeneveld CS, Jordan B, et al. Identification of Differential Tumor Subtypes of T1 Bladder Cancer. Eur Urol. 2020 Oct;78(4):533-537.


