According to the AUA guidelines, imaging is not used for the initial diagnosis of bladder cancer, however, bladder masses are often incidentally seen on CT imaging. The gold standard for diagnosis remains cystoscopy followed by examination under anesthesia and TURBT for pathologic confirmation of disease. The objective of imaging for newly diagnosed bladder cancer is to assess the extent of local tumor invasion, assess spread to the lymph nodes, and to evaluate the upper urinary tract and other organs. Imaging is unable to identify microscopic disease and local spread. However, newer techniques are improving pretreatment staging, the ability to predict early response to treatment and provide noninvasive alternatives to cystoscopy for those requiring long-term surveillance.
Computed tomography delivered in a split bolus technique is typically reserved for those <50 years of age. This includes:
- Pre-contrast: to assess the top of the kidneys to the base of the bladder
- First-IV contrast injection: 80 cc of Omni 300 at 2 cc/second, followed by a 25 cc saline chaser
- IV hydration between injections of 150 cc 0.9% saline infused over 0.5 cc/second
- Second IV contrast injection with 60 cc of Omni 300 @ 2 cc/second, followed by a 25 cc saline chase scan after 120 second diaphragm to base of the bladder evaluation
A three-phase CT scan is typically reserved for patients >50 years of age who are more likely to have significant pathology on imaging. This includes:
- Pre-contrast: to assess the diaphragm to the base of the bladder
- Contrast injection of 150 cc Omni at 3cc/second
- 90 second delayed imaging from the diaphragm to the ischial tuberosities
- 10 min delayed imaging from the diaphragm to the ischial tuberosities
However, CT has difficulty identifying flat lesions, carcinoma in situ, tumors <1 cm, and can be hampered by recent resection, biopsy, inflammation, systemic chemotherapy, and intravesical therapies.
Virtual cystoscopy is a three-dimensional modeling technique using CT or MRI images, which provides an indirect visualization of the mucosa providing a simulation of an endoscopic evaluation. The advantages of virtual cystoscopy are that it is noninvasive and allows visualization of difficult to access areas of the bladder (bladder neck and mucosa in diverticula). Disadvantages include an inability to obtain pathology and low sensitivity in identifying smaller tumors (<1 cm), flat lesions, and CIS.
The steps to virtual cystoscopy are as follows:
- The bladder should be adequately distended at the time of the procedure, but devoid of urine
- Imaging should be obtained in both the supine and prone positions
- Systematic inspection of the bladder starts by keeping the center of the bladder as the region of interest and moving systematically from normal to abnormal areas/surfaces (in grossly trabeculated bladders, small papillary growths may be overlooked)
Previous studies have shown that there is excellent interobserver agreement for CT images and suggest that virtual cystoscopy is more accurate than MRI as a source of imaging. Furthermore, accuracy has been assessed at 93% compared to cystoscopy, and tumors >5 mm can be detected by virtual cystoscopy.
MRI is an important modality for bladder cancer staging, providing excellent soft-tissue resolution and MPR capabilities. The accuracy for detecting NMIBC versus MIBC is ~85% and accuracy for assessing organ-confined disease is 82%. Newer techniques include dynamic contrast-enhanced MRI and diffusion-weighted MRI is better than T2WI for organ-confined higher stage tumors (sensitivity 98%; PPV 100%). Benefits of DWI are an increased lesions conspicuity, easier visualization of nearby lymph nodes, and an ability to compliment contrast-enhanced imaging in preoperative staging.
The Vesical Imaging Reporting and Data System (VI-RADS) was created by Dr. Valeria Panebianco et al through consensus using the existing literature, whereby standardizing bladder multiparametric MRI for research and clinical use. VI-RADS provides a systematic approach to reporting bladder MRI and defines risk of muscle invasion, and is applicable to untreated patients, as well as those having received a diagnostic TURBT.1 As follows is a schematic illustration of mpMRI appearances of VI-RADS scores 1-5 using DCE, MRI, DWI, and ADC: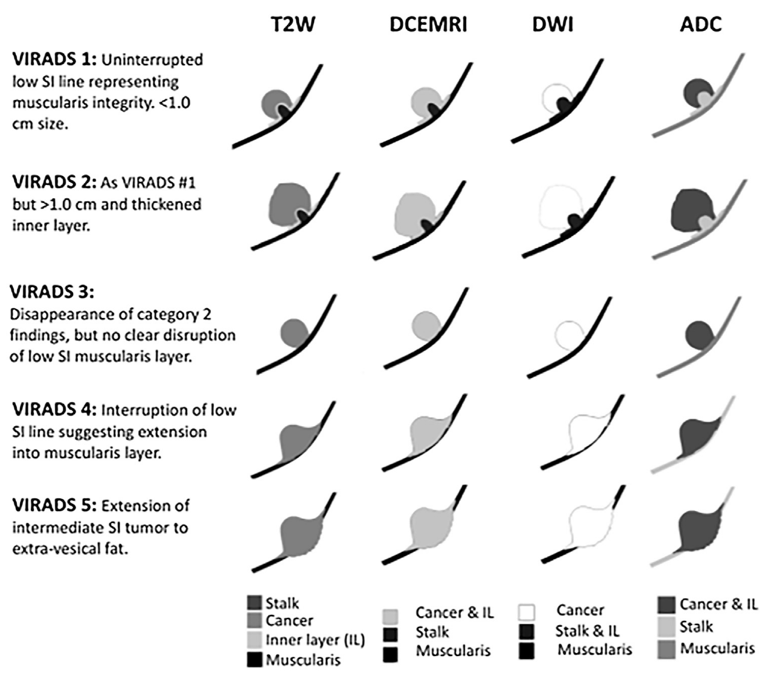
PET/CT imaging utilizes 18F-fluorodeoxyglucose and allows the detection of metabolic changes prior to anatomical changes in tissues. However, it is difficult to use given the urinary excretion of the isotope. Alternative tracers such as 11c-choline and 11c-methionine are not excreted in the urine, but there is limited experience using these tracers.
Artificial intelligence and machine learning have also started to make their way into the staging of bladder cancer space, with early models using machine learning developed to stratify tumors into stage <T2 versus >T2. Several radiomics-based predictive models have been developed for prediction of lymph node metastasis, pathologic grade of disease, and to distinguish between disease with and without complete response to chemotherapy.
Ultrasound is not routinely used for staging but is used for assessing hydronephrosis and investigating hematuria in the upper tracts (ie. renal masses). Contrast-enhanced ultrasound has been shown to be superior to conventional ultrasound imaging in differentiating NMIBC from MIBC, with early reports suggesting an accuracy of 88.4% and sensitivity of 94.7% for tumors >5 mm. However, lesions <5 mm is associated with only a sensitivity of 20% and a NPV of 28.6%. Contrast-enhanced ultrasound utilizes microbubbles consisting of high molecular weight gas bubbles stabilized by a shell of biocompatible material (protein, lipid, surfactant, polymer). With a diameter less than 10 um, there is no embolization of the capillaries and these bubbles are small enough to ensure pulmonary passage out of the body. For example, Sulphur hexafluoride SF6 has a mean terminal half-life is 12 minutes, with a half-life elimination of less than 1 minute, and with 98% of SF6 exhaled within 2 minutes.
Several studies have been published assessing initial experiences with contrast-enhanced ultrasonography. Li et al.2 evaluated the value of contrast-enhanced ultrasonography in the differentiation of high and low-grade urothelial carcinoma. Among 192 bladder lesions, there were 110 high-grade urothelial carcinoma and 82 low-grade urothelial carcinoma. At the time of contrast-enhanced ultrasonography, the prospective differentiation of bladder tumors showed sensitivity of 86%, specificity of 90%, accuracy of 88%, PPV of 92%, and NPV of 82% for high-grade tumors. For low-grade tumors, sensitivity was 85%, specificity was 89%, accuracy was 88, PPV was 85%, and NPV was 89%. Gupta et al.3 evaluated contrast-enhanced ultrasound as a modality to predict T stage and to predict the grade of the tumor preoperatively in 110 patients. In this study, contrast-enhanced ultrasound had a sensitivity of 75%, 65%, and 90%, and specificity of 95%, 85%, and 92% in detecting Ta, T1, and muscle invasion, respectively. Furthermore, contrast-enhanced ultrasound had a sensitivity of 78% and specificity of 85% for correctly detecting the grade of the lesion. Subsequently, Guo et al.4 used contrast-enhanced ultrasound to retrospectively assess the ability to differentiate between low-grade and high-grade bladder urothelial carcinoma in 105 patients. They found that the time from peak to one-half the signal intensity of the high-grade group was significantly longer than that of the low-grade group, and the descending slope was lower. The cutoff points of time from peak to one-half the signal intensity and descending slope for differentiating low-grade and high-grade bladder urothelial carcinoma were 48.06 seconds and 0.15 dB/seconds, respectively.
Dr. Dighe concluded her presentation with the following key take-home messages:
- CT is the mainstay of assessment in locally advanced and metastatic disease, but there are limitations in assessing the primary tumor
- Two dimensional techniques have no role in route assessment of the primary tumor, however, the presence of hydronephrosis is suggestive of muscle-invasive bladder cancer. Contrast-enhanced and three-dimensional techniques are under investigation
- MRI is useful in identifying muscle-invasive and extravesical disease. Functional MRI is currently under investigation as a predictive tumor biomarker
- Virtual cystoscopy utilizes CT or MRI data to reconstruct the bladder mucosa and simulate endoscopic evaluation, but unlike for other sites such as the gastrointestinal tract, it is not used routinely
- FDG-PET/CT is not used as an initial staging modality, but is often used in conjunction with other imaging if uncertainty exists; FDG use for staging local disease is limited predominantly by urinary excretion. Alternative isotopes and receptor-specific molecules are under investigation
Presented by: Manjiri Dighe, MD, MBBS, University of Washington School of Medicine, Seattle, WA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md during the 4th annual bladder cancer translational research meeting, co-sponsored by the American Urological Association (AUA) and the Johns Hopkins Greenberg Bladder Cancer Institute, March 4-6, 2021
References:
- Panebianco V, Narumi Y, Altun E, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur Urol2018 Sep;74(3):294-306.
- Li Q, Tang J, He E, et al. Differentiation between high- and low-grade urothelial carcinomas using contrast enhanced ultrasound. 2017 Aug 10;8(41):70883-70889.
- Gupta VG, Kumar S, Singh SK, et al. Contrast enhanced ultrasound in urothelial carcinoma of urinary bladder: An underutilized staging and grading modality. Cent European J Urol. 2016;69(4):360-365.
- Guo S, Xu P, Zhou A, et al. Contrast-Enhanced Ultrasound Differentiation between low- and high-grade bladder urothelial carcinoma and correlation with tumor microvessel density. J Ultrasound Med. 2017 Nov;36(11):2287-2297.


