Figure 1 – PET/CT imaging in the diagnosis of prostate cancer:

According to the European Association of Urology (EAU) guidelines, the role of PET imaging is divided into lymph node staging and metastasis staging. In the lymph node staging, PET-Choline has good specificity for lymph node metastasis (92%), with a sensitivity of 62%. In intermediate/ high-risk patients, there are contradictory results for choline PET/CT and whole-body MRI. Overall, Choline PET/CT does not reach clinically acceptable diagnostic accuracy for the detection of lymph node metastases and cannot rule out a nodal dissection based on risk factors or nomograms. A published metanalysis has shown that for lymph node staging, choline-PET/CT has a sensitivity and specificity of 86% at the patient level, with a large 95% CI of 3-100%.
Furthermore, it has a sensitivity and specificity of 80% and 97%, respectively, at the lesion level. For the metastases staging, choline-PET/CT has a lower sensitivity and a higher specificity than whole-body MRI. These are very limited data on its role in metastases staging, being able to change management in probably 20-25% of cases, but it is not known whether there is any correlation with the outcomes.
Choline is a key precursor in the biosynthesis of phosphatidylcholine, a major component of the cell membrane. It is quite an old tracer, discovered almost 20 years ago, and it depicts the membrane metabolism of choline. The labeled variants include C-11 and F-18. When there is a slow proliferation of prostate cancer cells, there is a slow membrane metabolism, leading to a small amount of choline uptake. Its sensitivity is low in low volume disease and low PSA levels.
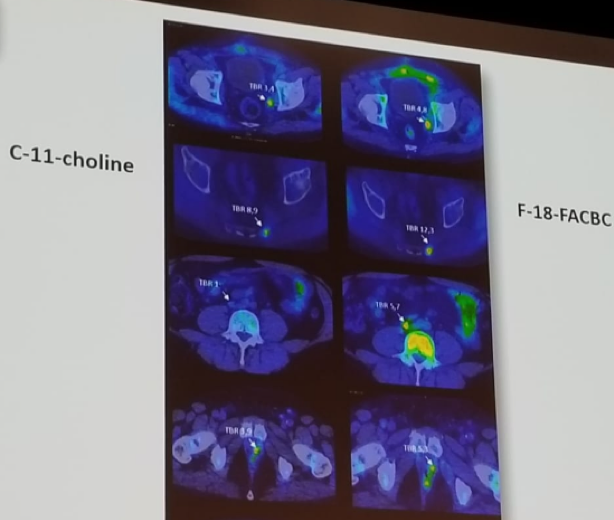
The next discussed tracer used in prostate cancer was FACBC (Fluciclovine). This is a synthetic L-Leucine analog, developed 15 years ago for imaging of brain tumors. Its first report in prostate cancer was in 2007. It depicts the functional activity of two different transmembrane amino acid transporters (ASCT2 and LAT1). There is a favorable distribution of this tracer in the body: its uptake in the kidney is negligible, and there is no activity of this tracer in the urinary tract. It may have improved detection rate as compared to choline PET/CT when PSA levels are low. The detection of prostate cancer recurrence is based on elevated blood PSA levels following prior treatment. A comparison of images from Fluciclovine and choline-PET/CT is shown in figure 2, demonstrating a lesion diagnosed with Fluciclovine, but not diagnosed with choline (3rd line).
Figure 2 – Comparison of choline and fluciclovine PET/CT imaging:
Next, the prostate-specific membrane antigen (PSMA), which is the new “wonder” tracer was discussed. PSMA is a type 2 membrane-bound glycoprotein. It is expressed in all forms of prostate tissue and is over-expressed in carcinoma. It is also found in the neovascular tissue of most solid tumors. The PSMA has several derivatives, and it is convenient to label it with Ga-68 or F-18 for PET/CT. It has rapid background clearance, high targeting, and early imaging. There has been a tremendous amount of data that has been published so far on the use of this tracer in recurrent prostate cancer. Figure 3 demonstrates a suspicious node that has lighted up on the left side with PSMA PET/CT.
Dr. Oyen concluded his discussion by stating that Ga-68 PSMA PET/CT imaging is a major step forward in the detection of disease. It has improved lesion detection rate, and it can detect lesions sized down to 4 mm. However, it cannot detect micrometastatic disease accurately, and other non-prostate cancers also express PSMA.
In any case, we still do not even know the answer to the question whether earlier and more appropriate diagnosis of small volume disease improves the patient’s outcomes. Unfortunately, conventional development of tracers for imaging through large, randomized phase 3 studies are unlikely to occur.
Figure 3 – PSMA PET/CT imaging showing a positive node:

Presented by: Wim Oyen, London, Great Britain
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2nd EAU Update on Prostate Cancer (PCa18)– September 14-15, 2018 – Milan, Italy


