(UroToday.com) The 2024 PSMA conference featured a presentation by Dr. Robert Flavell discussing the background for PSMA imaging. Dr. Flavell started by highlighting that PSMA targeted imaging and therapy is a game changing theranostic strategy owing to high target expression across the prostate cancer disease course.
PSMA PET is the standard of care for imaging of newly diagnosed high risk prostate cancer and in biochemical recurrence, as well as for treatment selection in post-chemotherapy metastatic castration resistant prostate cancer (mCRPC) patients.
The prostate specific membrane antigen is a 100 kDa transmembrane type II glycoprotein that is highly expressed in prostate cancer, and to a lesser extent the neovasculature of other tumors, normal prostate, and other epithelia. The PSMA protein may be targeted with small molecules against the enzymatic active site, as well as with antibodies (usually against the extracellular domain). As a prostate cancer theranostic prostate cancer target, small molecule agents are primarily focused around the Glu-Urea-Lys based scaffold (shown in red):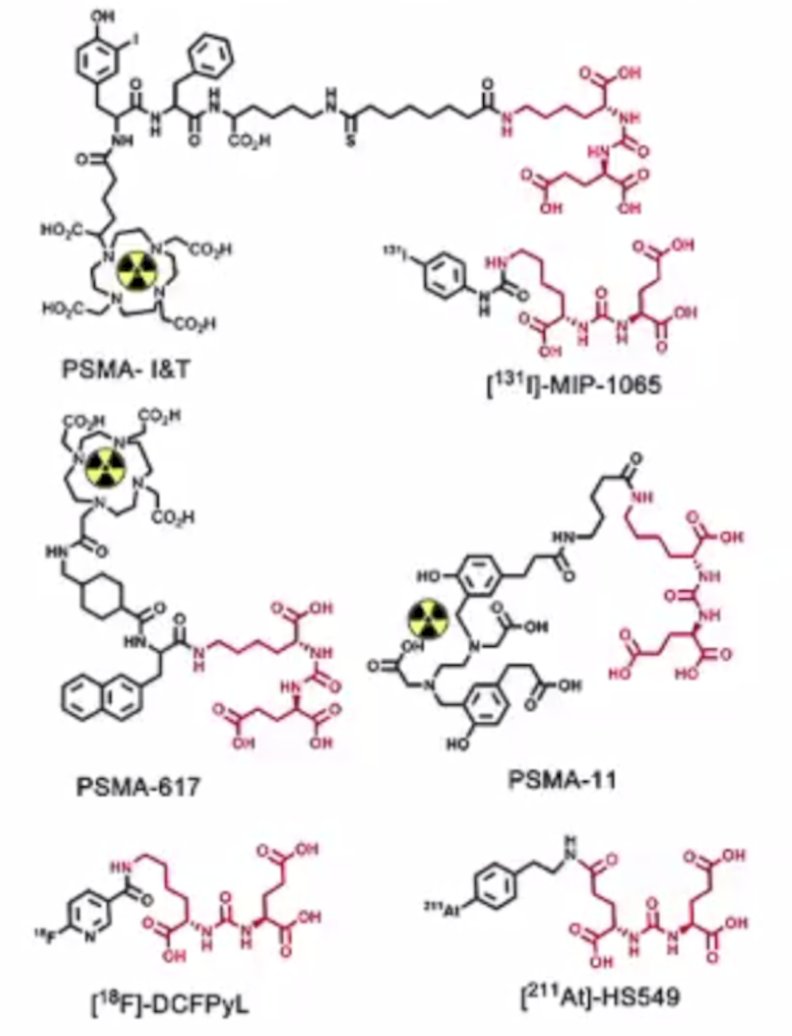
Lysine may be appended with a variety of substituents to achieve the desired imaging or therapeutic outcome. Additionally, positron emitting isotopes may also be appended for imaging (ie. alpha or beta emitters for therapy), and changes in imaging and therapeutic payload can have a substantial impact on affinity and delivery.
Dr. Flavell notes that the prostate cancer disease continuum offers many opportunities for PSMA PET/CT imaging:
- Initial diagnosis: prior to definitive therapy (radiation therapy, radical prostatectomy) to detect metastasis
- Biochemical recurrence: utilized to assess localized recurrence, providing opportunities for metastasis directed therapy, salvage radiation, or hormonal therapy
- Castration resistant prostate cancer: providing opportunities for therapies such as hormonal/ARSIs (ie. abiraterone, enzalutamide), chemotherapy (ie. docetaxel, cabazitaxel), immunotherapy (ie. sipuleucel-T), nuclear therapy (ie. radium-223), and clinical trials
- Post-chemotherapy mCRPC: assessing eligibility for 177Lu-PSMA-617

It is important to understand the normal biodistribution for PSMA PET, which may include the lacrimal glands, liver, spleen, kidneys, ureter, bladder, small bowel, etc. Less commonly understood is the physiologic celiac ganglion uptake:1
Additional challenging locations may include presacral ganglion and stellate (cervical) ganglion: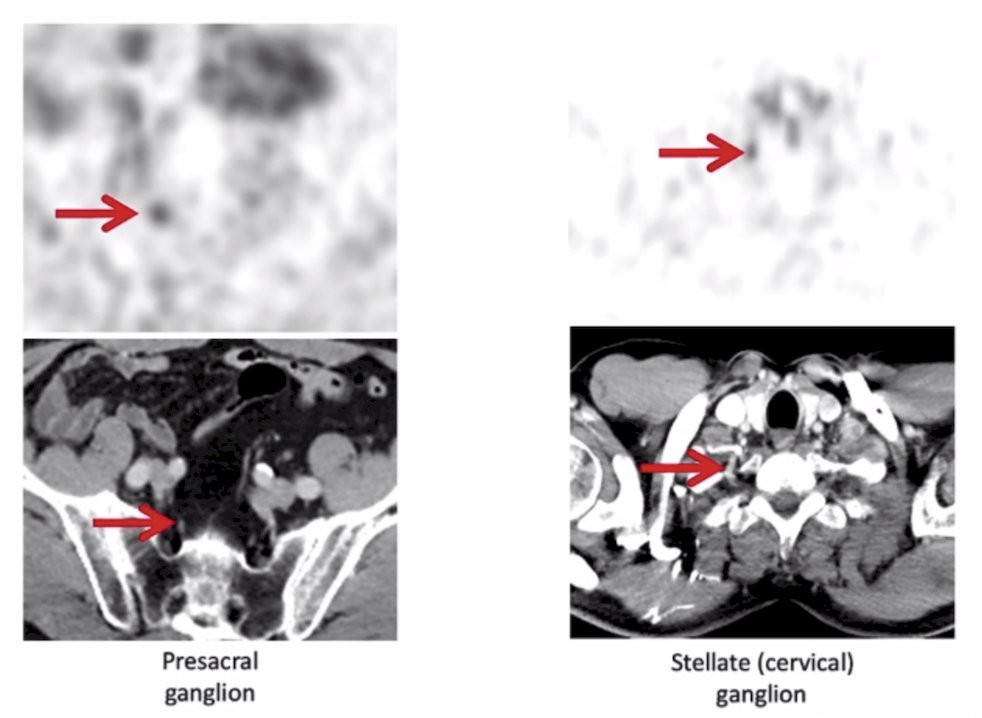
Typical patterns of disease detected by PSMA PET/CT include the prostate, oligometastatic bone lesions, pelvic and retroperitoneal nodes, and diffuse bone metastases: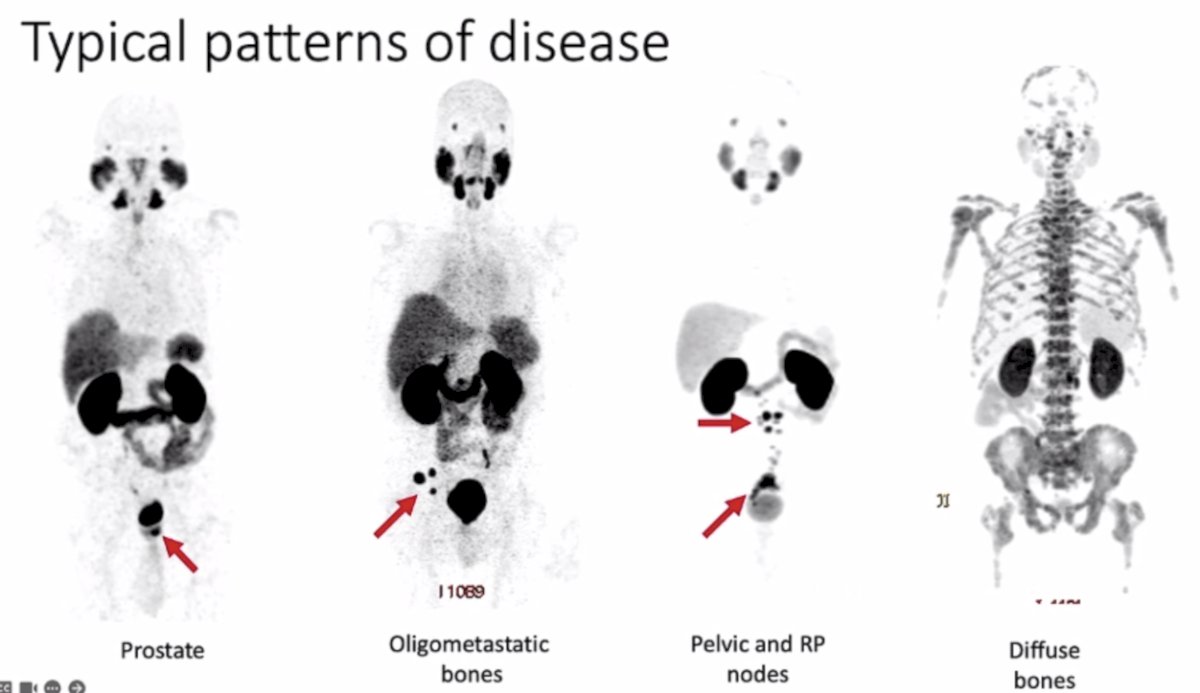
Dr. Flavell concluded his presentation discussing the background for PSMA imaging with the following take-home points:
- PSMA is expressed in most prostate cancers
- The Glu-Urea-Lys pharmacophore allows targeting of PSMA, using diagnostic PET imaging and therapeutic isotopes
- PSMA PET is standard of care for initial staging in high risk prostate cancer and at biochemical recurrence
- PSMA treatment with 177Lu-PSMA-617 with imaging selection is a new standard of care option in post-chemotherapy mCRPC
- Common patterns of disease on PSMA PET include prostate, lymph node, and bone metastases, with other sites typically occurring later
Presented by: Robert Flavell, MD, PhD, UCSF, San Francisco, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 PSMA Conference, San Francisco, CA, Thurs, Jan 18 – Fri, Jan 19, 2024.
Related content: PSMA Targeted Imaging and Therapy in Prostate Cancer "Presentation" - Robert Flavell
References:
- Krohn T, Verburg FA, Pufe T, et al. [(68)Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: An important pitfall in clinical practice. Eur J Nucl Med Mol Imag. 2015 Feb;42(2):210-214.


