(UroToday.com) The 2024 PSMA conference featured a presentation by Dr. Wolfgang Fendler discussing whether the differences in PET agents matter. Dr. Fendler started by asking “How many radioligands do we actually need?” Indeed, there are several agents, including (i) 68Ga-PSMA-11, (ii) 68Ga-PSMA-I&T, (iii) 18F-DCFPyL, (iv) 99Tc-MIP1404, (v) 18F-PSMA-1007, (vi) 64Cu-PSMA-617, (vii) 89Zr-PSMA-617 (viii) 18F-rhPSMA-7.3: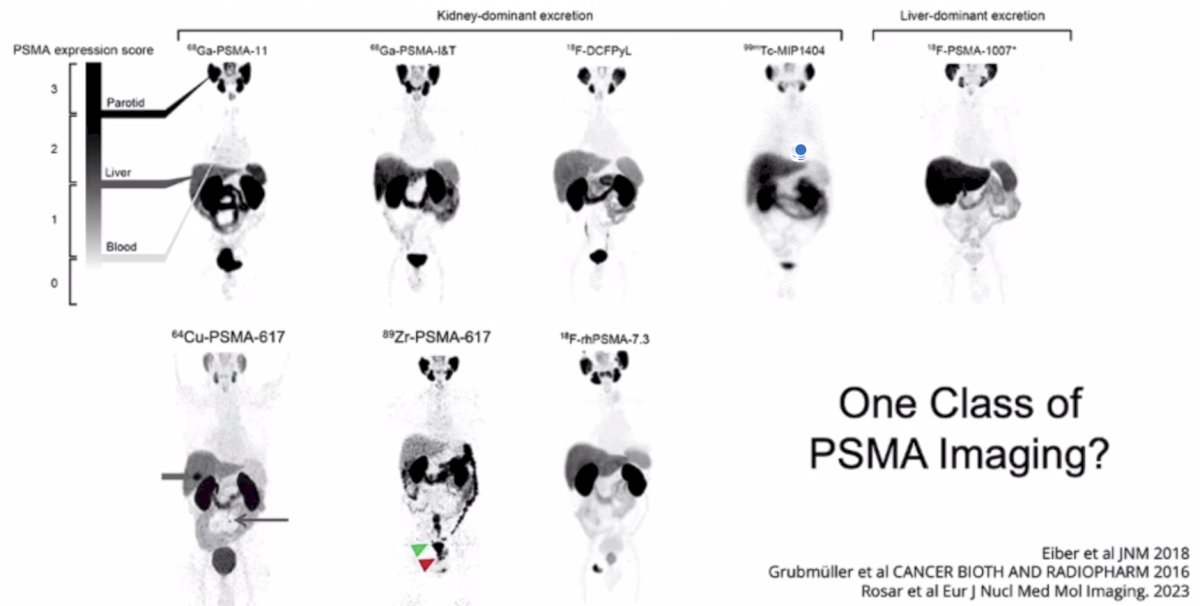
According to Dr. Fendler, there are three components to assessing if different PET agents matter:
- Logistics – ie. radiopharmacy
- Facts – ie. evidence
- Experience – ie. clinical application
To assess optimizing radiopharmacy costs, Dr. Fendler discussed the Essen, Germany experience. Until 2021, this encompassed utilization of 68Ga, including three daily [68Ga]Ga-PSMA-11 productions using generator/module based synthesis (15 per week). Depending on the age of a 68Ga-generator, and a production interval of 3 hours, this led to a yield of 1.0 to 0.6 GBq per batch. This corresponds to 9 patient doses per day (45 per week), with an uncertainty in case of delay (given the 68 minute half-life), and 15 dose shipments per week. As such, the above model corresponds to 60 FTE hours per 45 doses. From 2022 onward, this encompassed 18F in Essen, including two [18F]Ga-PSMA-1007 productions using cyclotron/module based synthesis (2 per week). This led to a stable yield of 60 to 80 GBq per batch, corresponding to 30 patient doses per day (60 per week), with flexible scan times (given the 110 minute half-life), and 6 dose shipments per week. As such, the above model corresponds to 22 FTE hours per 60 doses, which is certainly more cost effective.
In a study assessing head-to-head comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in staging prostate cancer using histopathology and immunohistochemical analysis as a reference standard, Kuten et al. identified PSMA-avid lesions in the prostate among 16 patients with an almost perfect concordance between the two tracers (kappa range: 0.87 – 1.0).1 Importantly, the dominant intraprostatic lesions were similarly detected by both radiotracers, and a second less intense positive focus was detected in 4 patients only with 18F-PSMA-1007: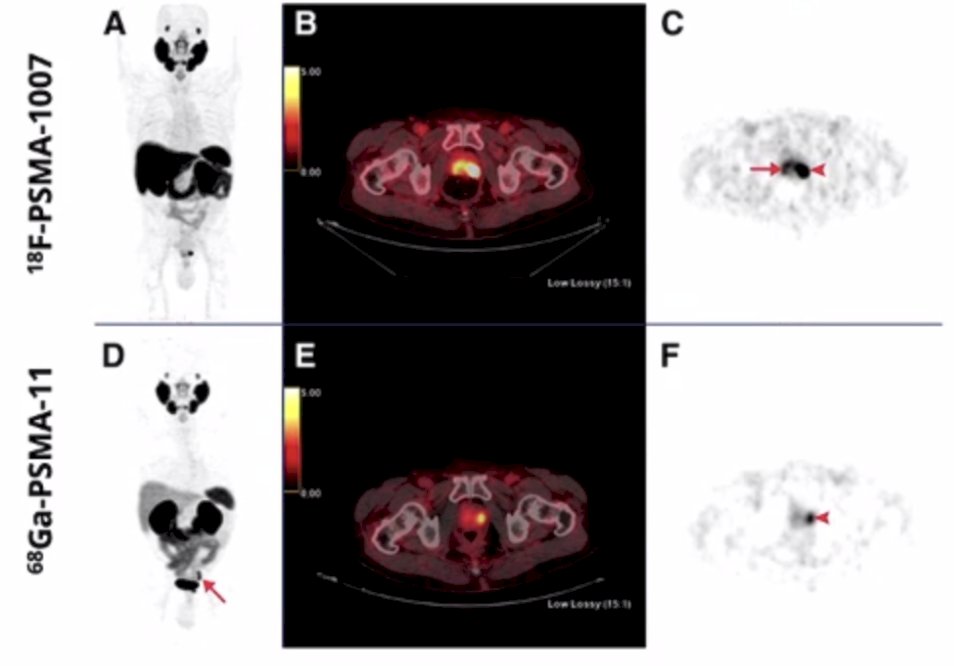
Another study from Pattison et al. prospectively assessed an intra-individual blinded comparison of [18F]PSMA-1007 and [68Ga]Ga-PSMA-11 PET/CT imaging among 50 men.2 Overall, the AJCC prognostic stage was concordant in 92% of patients, with two patients upstaged for both [18F]PSMA-1007 and [68Ga]Ga-PSMA-11. Furthermore, [18F]PSMA-1007 had more equivocal results (one regional node, six equivocal bone lesions, one of which was subsequently confirmed metastatic) than [68Ga]Ga-PSMA-11 (one equivocal recurrence):![[68Ga]Ga-PSMA-11](/images/com-doc-importer/142-psma-pet-and-rlt-2024/psma-pet-and-rlt-2024-do-the-differences-in-pet-agents-matter/image-2.jpg)
In the biochemical recurrence population, De Man et al. performed a prospective double-blind randomized cross-over trial assessing 18F-PSMA-11 versus 68Ga-PSMA-11 PET/CT for staging of biochemical recurrence.3 The following flow chart shows the randomized crossover design for this trial:
This study found that the 18F-PSMA-11 tracer is non-inferior to 68Ga-PSMA-11, and the primary endpoint was met: per patient, the proportions of positive scans rated by three readers were 67%/67%, 65%/65%, and 73%/70% for 18F-PSMA-11/68Ga-PSMA-11 PET/CT. Based on the aforementioned studies, a systematic review and meta-analysis was recently published in 2023 comparing 18F-based PSMA radiotracers with 68Ga-PSMA-11 PET/CT.4 Overall, [18F]-DCFPyL was observed to have a similar lesion detection rate to [68Ga]Ga-PSMA-11 with no increase in false positive rates. Additionally, [18F]-PSMA-1007 was found to have a greater local lesion detection rate because of its predominant hepatobiliary excretion route. However, [68Ga]Ga-PSMA-11 was observed to have a similar local lesion detection rate in studies that administered furosemide prior to the scan. Finally, [18F]-PSMA-1007 was found to have a significant number of benign bone lesions showing uptake:
Recently, Dr. Fendler’s group assessed unspecific 18F-PSMA-1007 bone uptake evaluated through PSMA-11 PET, bone scanning, and MRI triple validation in patients with biochemical recurrence.5 For this study, all patients (n = 383) who underwent 68Ga-PSMA-11 PET and all patients (n = 409) who underwent 18F-PSMA-1007 PET due to biochemical recurrence were included for an interindividual comparison of bone metastases and unspecific bone uptake rate. In a second analysis, they regarded all patients with unspecific bone uptake in 18F-PSMA-1007, characterized by focal bone uptake with an SUVmax > 4 and PSA ≤ 5 ng/mL, who underwent additional 68Ga-PSMA-11 PET (n = 17) (interindividual comparison). Of these, 12 patients also had bone scintigraphy and whole-body MRI within a 1 to 5 week interval. Bone uptake seen on 18F-PSMA-1007, but not on any of the other four modalities (CT, MRI [n = 1], bone scanning, and 68Ga-PSMA-11 PET) was recorded as false-positive. As follows is the anatomic distribution of unspecific bone uptake see on 18F-PSMA-1007 PET/CT, notably for distribution in the ribs and pelvis: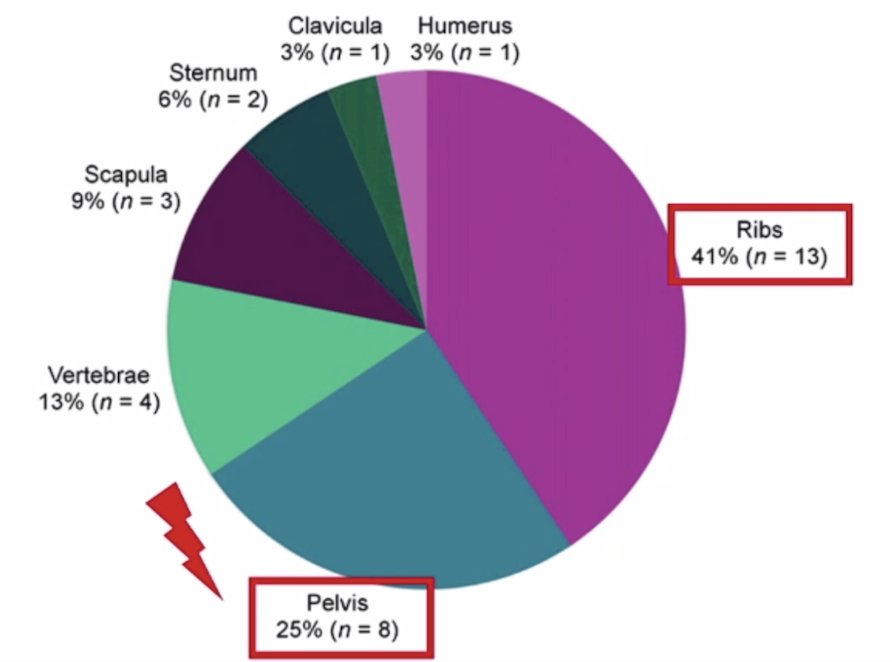
Additionally, there was no significant difference in the percentage of M1b detection between these tracers in routine clinical practice: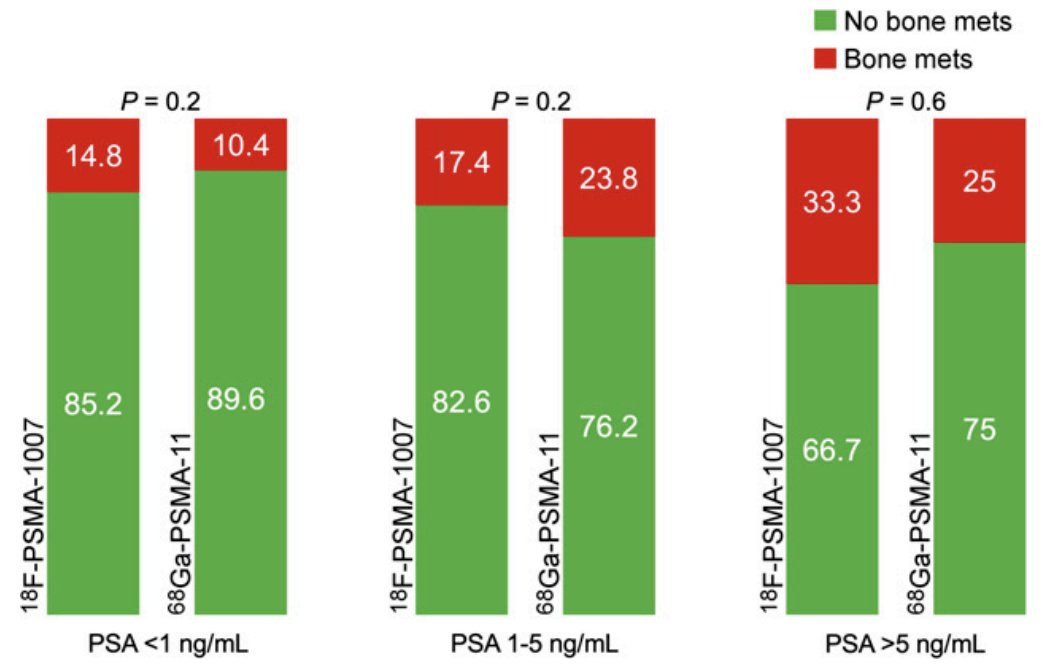
Thus, the recommendations for clinical interpretation of single/oligo 18F-PSMA-1007 or 18F-rhPSMA bone uptake are (i) to avoid over-sensitive interpretation of the ribs, and (ii) to correlate with CT and consider the PSA level.
Dr. Fendler then highlighted several important trials in the disease space that are ongoing. The PROTEUS study is assessing PSMA-PET imaging at 3 months post adjuvant treatment, at biochemical failure, and every 6 months until distant metastasis or death. Importantly, in this trial, all of 18F-PSMA-1007, 18F-DCFPyL, 18F-PSMA-11, and 68Ga-PSMA-11, are allowed. The trial schema is as follows: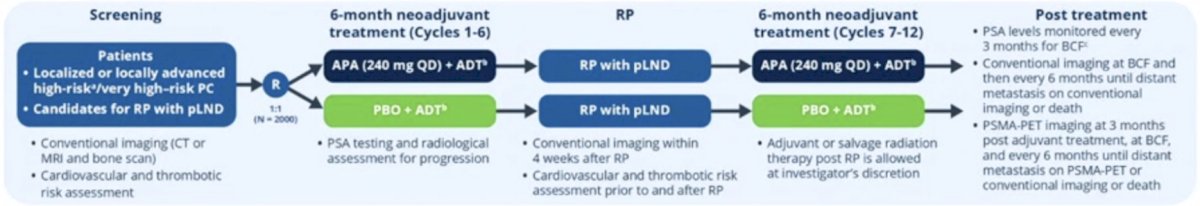
The second trial Dr. Fendler discussed was the PRIMORDIUM trial for patients with >=1 locoregional lesions on PSMA-PET and high risk biochemical recurrence, imaged with a PSMA PET at screening, 6 months, 12 months, and then annually or until the PSA >= 0.2 ng/mL. Again, in this trial, all of 18F-PSMA-1007, 18F-DCFPyL, 18F-PSMA-11, and 68Ga-PSMA-11, are allowed. The trial design for PRIMORDIUM is as follows: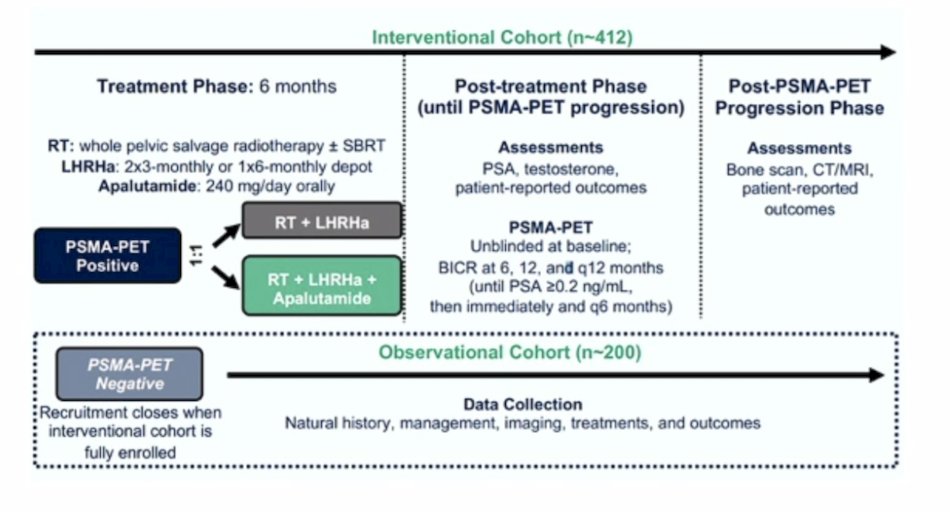
Finally, Dr. Fendler noted that the ASCO Guideline panel just recently recommended that either 68Ga-PSMA-11, 18F-DCFPyL or 18F-rhPSMA can be used as radiotracers to determine eligibility.
Dr. Fendler concluded his presentation discussing whether the differences in PET agents matter with the following take-home points:
- The evidence does not support clinical impact of radioligand choice
- PSMA PET is one class of imaging, however, specific pitfalls exist, for example, ureter/bladder uptake for PSMA-11/DCFPyL and bone uptake for rhPSMA/PSMA-1007
- Knowledge of physiologic distribution and common pitfalls is critical for PSMA PET interpretation
- PSMA-1007 delivers superior local detection, however, rhPSMA/PSMA-1007 bone uptake needs careful assessment to avoid equivocal findings
- Clinical trials should be inclusive for the different radioligands
Presented by: Wolfgang Fendler, MD, University of Duisburg-Essen and German Cancer Consortium, University Hospital Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 PSMA Conference, San Francisco, CA, Thurs, Jan 18 – Fri, Jan 19, 2024.
Related Content: Examining Differences Between PSMA PET Imaging Agents "Presentation" - Wolfgang Fendler
References:
- Kuten J, Fahoum I, Savin Z, et al. Head-to-Head Comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J Nucl Med. 2020 Apr;61(4):527-532.
- Pattison DA, Debowski M, Gulhane B, et al. Prospective intra-individual blinded comparison of [18F]PSMA-1007 and [68Ga]Ga-PSMA-11 PET/CT imaging in patients with confirmed prostate cancer. Eur J Nucl Med Mol Imaging. 2022 Jan;49(2):763-776.
- De Man K, Van Laeken N, Schelfhout V, et al. 18F-PSMA-11 versus 68Ga-PSMA-11 positron emission tomography/computed tomography for staging and biochemical recurrence of prostate cancer: A prospective double-blind randomized cross-over trial. Eur Urol. 2022 Nov;82(5):501-509.
- Huang S, Ong S, McKenzie D, et al. Comparison of 18F-based PSMA radiotracers with [68Ga]Ga-PSMA-11 in PET/CT imaging of prostate cancer – A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2003 Nov 28 [Epub ahead of print].
- Seifert R, Telli T, Opitz M, et al. Unspecific 18F-PSMA-1007 Bone Uptake Evaluated Through PSMA-11 PET, Bone Scanning, and MRI Triple Validation in Patients with Biochemical Recurrence of Prostate Cancer. J Nucl Med. 2023 May;64(5):738-743.


