(UroToday.com) The 2024 PSMA conference featured a presentation by Dr. Michael Morris discussing the VISION1 and TheraP2 trials. Dr. Morris started by discussing the VISION trial, an international, randomized, open-label phase III study evaluating 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with a next-generation androgen receptor signaling inhibition (abiraterone, enzalutamide, etc) and one or two prior lines of taxane chemotherapy.
Patients must have had an ECOG performance status of 0-2 and life expectancy of at least 6 months. Importantly, patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Furthermore, patients could have no PSMA-negative metastatic lesions. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks x 6 cycles) plus standard of care or standard of care alone. Standard of care treatments were at the discretion of the treating investigator; however, cytotoxic chemotherapy, immunotherapy, and radium-223 were explicitly excluded. The trial design for VISION is as follows: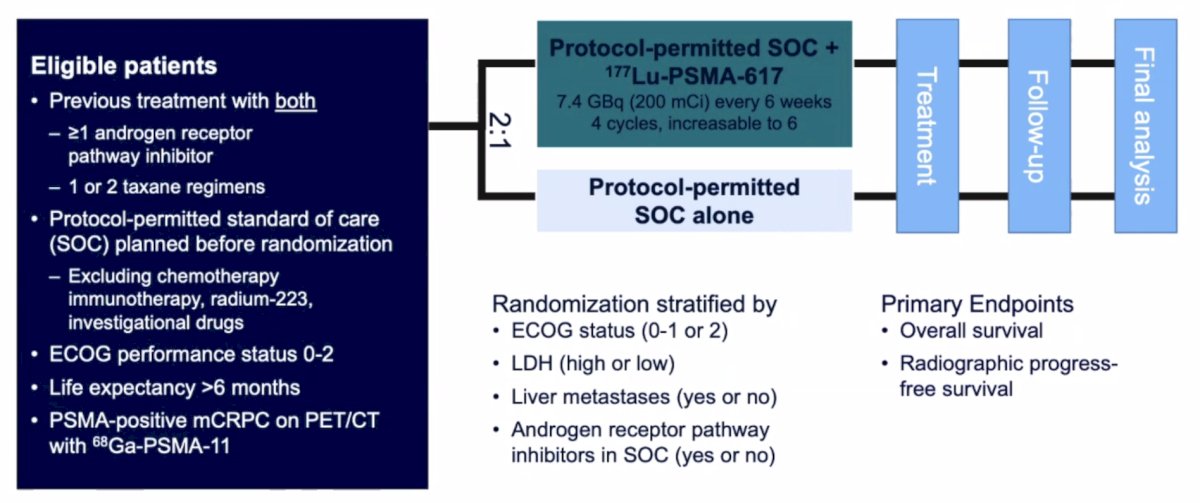
The authors assessed two alternate primary endpoints: (i) rPFS using the PCWG3 criteria by independent central review and (ii) overall survival. Secondary endpoints included ORR (RECIST v1.1), disease control rate, time to first symptomatic skeletal event, and safety/adverse event profile. VISION enrolled 831 patients, and in keeping with the 2:1 randomization schema, 551 patients were allocated to 177Lu-PSMA-617 + standard of care and 280 were allocated to standard of care only. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved OS by a median of 4.0 months (median OS: 15.3 vs 11.3 months; HR 0.62, 95% CI 0.52 to 0.74; p < 0.001, one-sided), compared to standard of care alone, in the overall cohort of all randomized patients: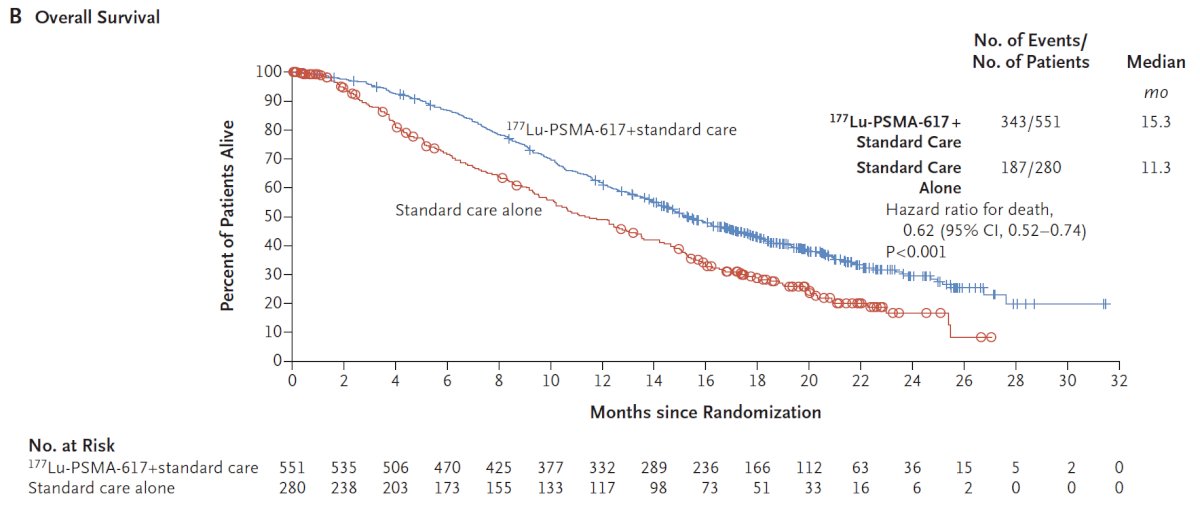
With regards to the other primary endpoint of rPFS, treatment with 177Lu-PSMA-617 + standard of care significantly improved rPFS by a median 5.3 months (median rPFS, 8.7 vs 3.4 months; HR 0.40, 99.2% CI 0.29 to 0.57; p < 0.001, one-sided):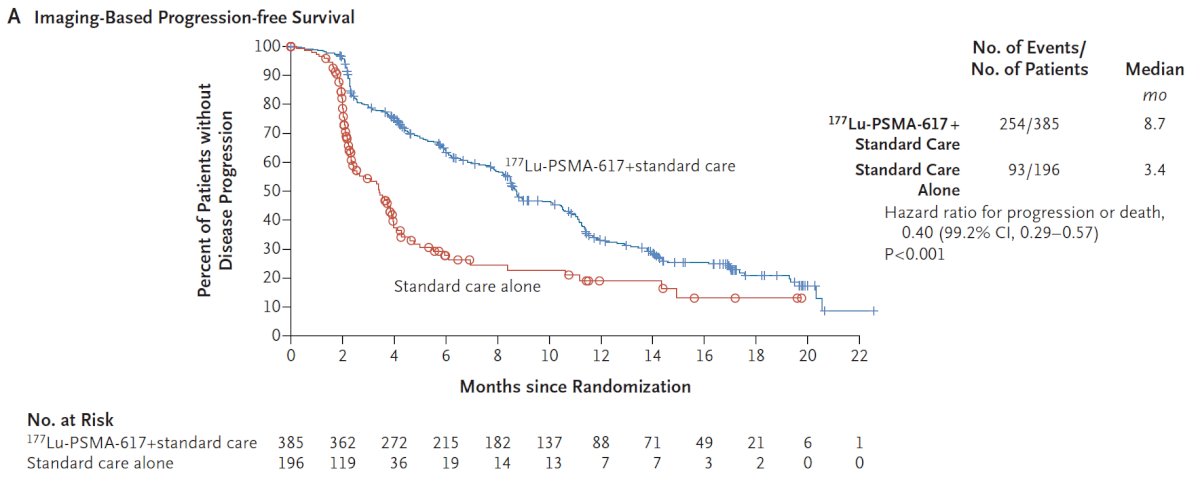
Similar to overall survival, rPFS subgroup analyses demonstrated a generally consistent effect, though conclusions were limited in many subgroups due to small numbers. In addition to these primary endpoints, the addition of 177Lu-PSMA-617 to standard of care statistically significantly improved all key secondary endpoints, including ORR (29.8% vs 1.7%), disease control rate (89.0% vs 66.7%) and time to first symptomatic skeletal event (median time: 11.5 vs 6.8 months; HR 0.50, 95% CI 0.40 to 0.62):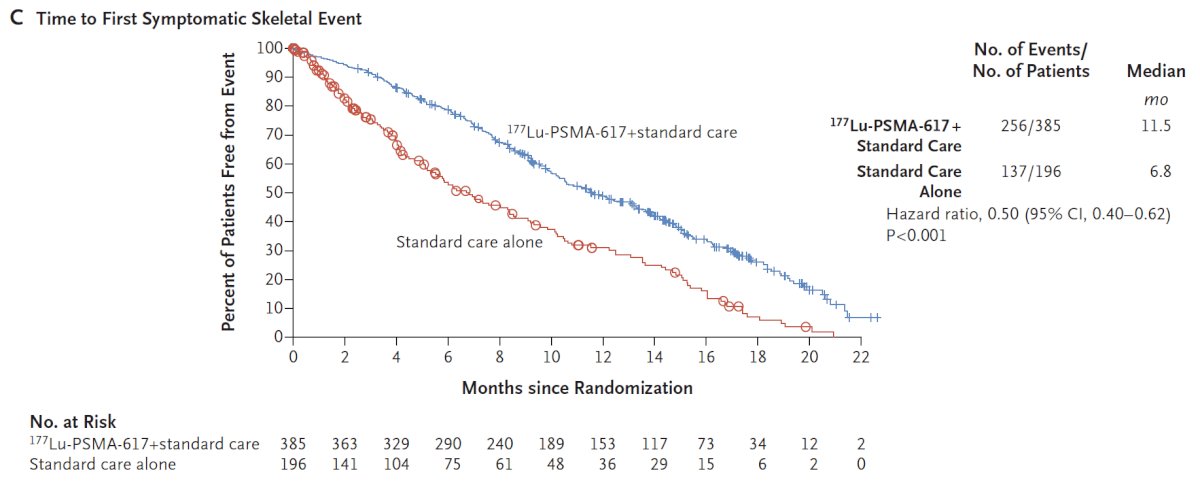
Time to worsening in health-related quality of life and pain were also improved with 177Lu-PSMA-617 + standard of care. This included the FACT-P total score (HR 0.46, 95% CI 0.35 – 0.61) and BPI-SF pain intensity (HR 0.45, 95% CI 0.33 – 0.60):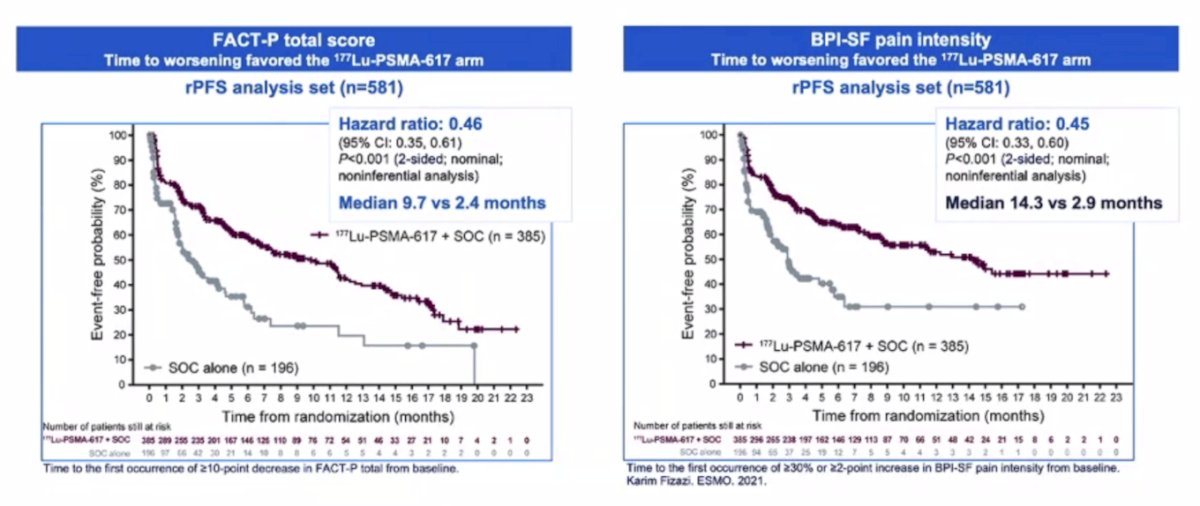
Further, PSA responses (whether defined as a 50% decrease or an 80% decrease) were significantly more common among those treated with 177Lu-PSMA-617 + standard of care: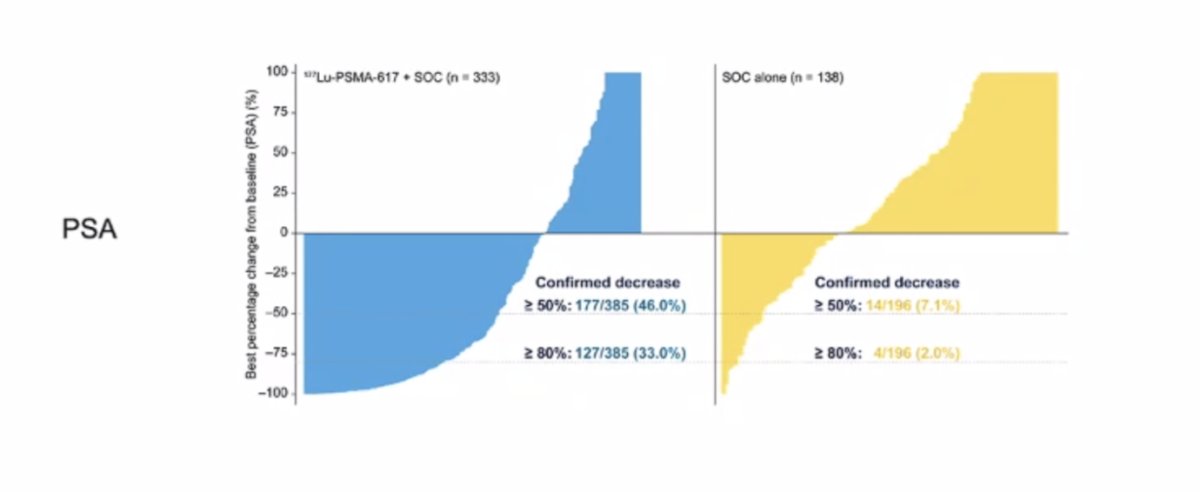
While a higher rate of high-grade (grade 3-5) treatment-emergent adverse events was observed with 177Lu-PSMA-617 (28.4% vs 3.9%) at the time of initial reporting, overall therapy was well tolerated. It is important to note that treatment exposure was more than three times longer in the 177Lu-PSMA-617 group than in the control group. Adjusted safety analysis, accounting for a longer safety observation due to longer rPFS in patients receiving 177Lu-PSMA-617, revealed a comparable incidence of treatment-emergent adverse events between arms. More than 50% of patients with mCRPC were able to receive 5 or 6 cycles (of a planned 6 cycle) of 177Lu-PSMA-617. Grade 3-5 treatment related AEs were seen in 60.4% of patients who received 4 or less cycles of 177Lu-PSMA-617 and 46.4% in those who tolerated 5-6 cycles: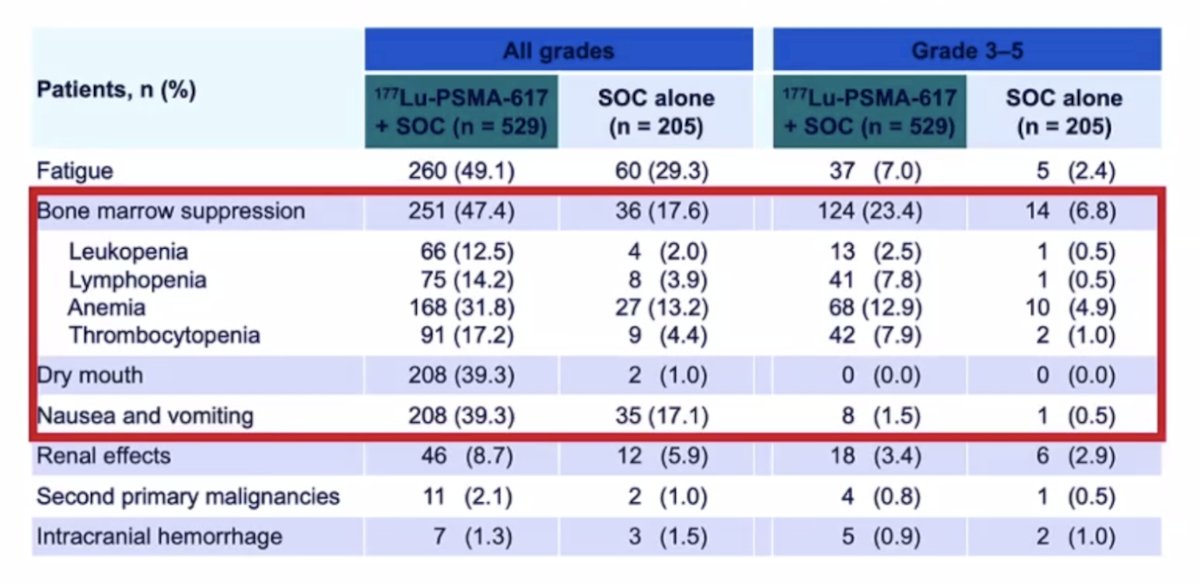
Presented at ASCO 2023, Dr. Ken Herrmann presented predictive models from VISION for clinical outcomes after 177Lu-PSMA-617. Data from 831 adults were analyzed, and in the initial univariate analyses, 76% of parameters assessed were prognostic for overall survival, 69% for rPFS, and 38% for PSA50. The OS model included 10 parameters and had a c-index of 0.73 (95% CI 0.70-0.76):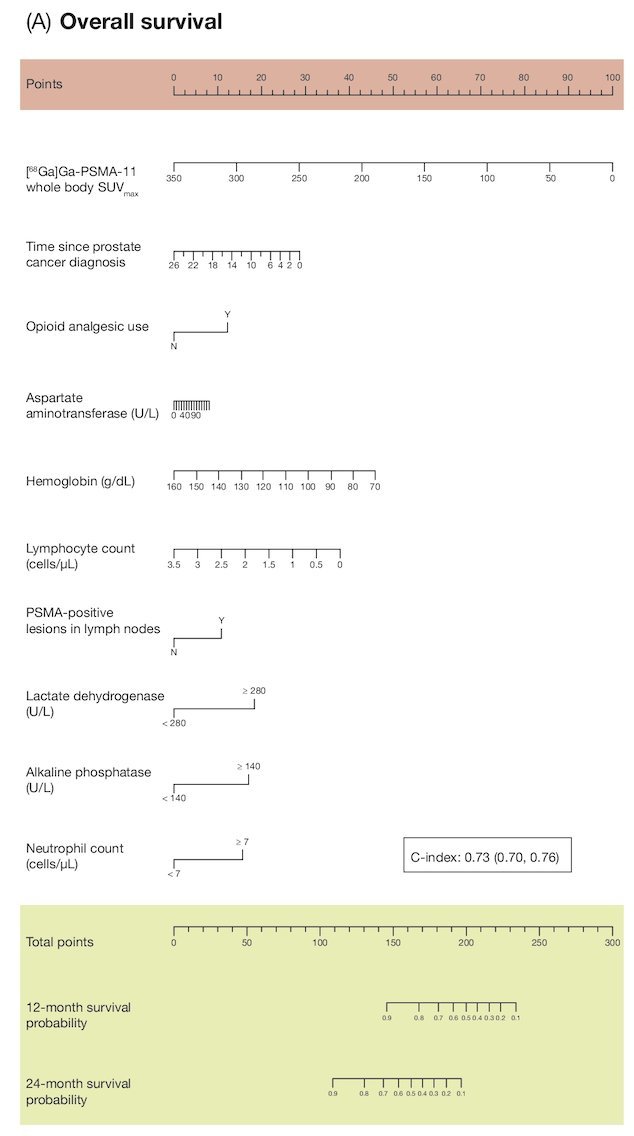
The multivariate model for rPFS included seven parameters and had a C-index of 0.68 (95% CI 0.65-0.72):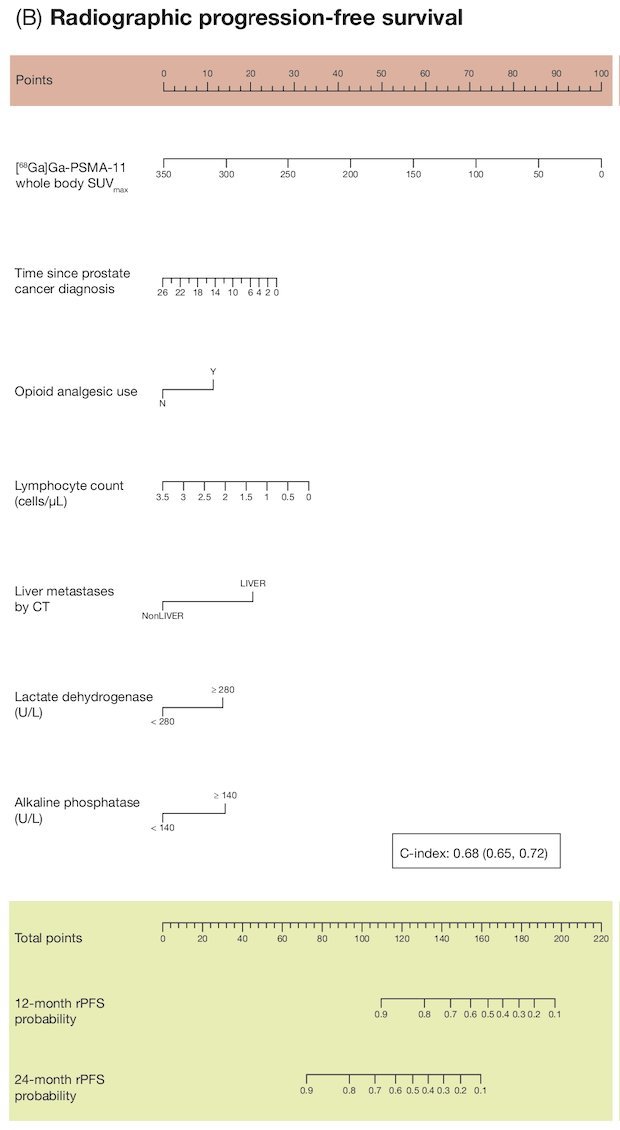
Dr. Morris notes that PSMA PET has a complex relationship to outcome. In data presented by Dr. Andrew Armstrong at ASCO 2022 assessing the prognostic value of baseline 68Ga-PSMA-11 PET imaging in men undergoing 177Lu-PSMA-617 in the VISION trial, higher whole-body SUVmean was associated with improved clinical outcomes: those patients in the highest quartile (SUVmean: rPFS, ≥ 10.2; OS, ≥ 9.9) had a median rPFS and OS of 14.1 and 21.4 months, vs 5.8 and 14.5 months for those in the lowest quartile (< 6.0; < 5.7), respectively: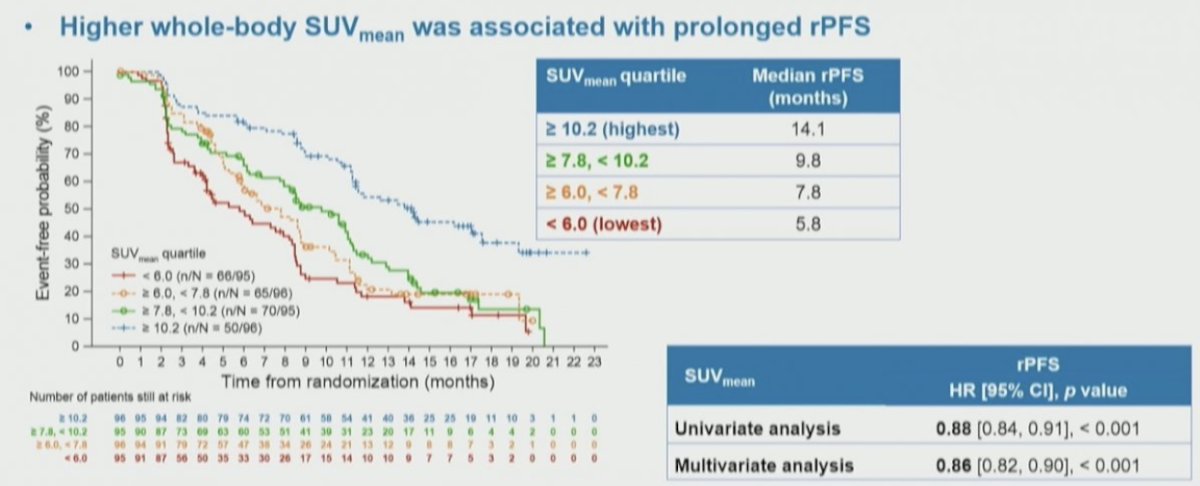
The association of these quartiles of whole body SUVmean was also assessed for overall survival, again finding a significant stratification between the groups. Notably, among these patients who received 177Lu-PSMA-617, those with the highest SUVmean levels had the longest overall survival: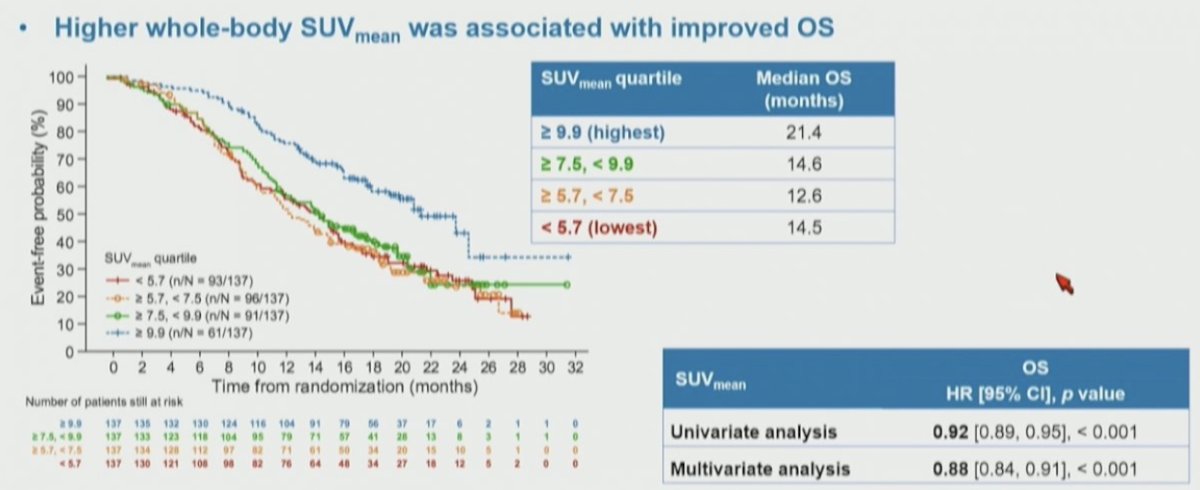
Dr. Morris cautions that the use of 177LuPSMA-617 therapy should not be based on SUVmean, noting that even in the lowest SUVmean patients, these patients still had greater benefit than those in the control group.
Dr. Morris then discussed the TheraP trial, which was the first randomized study to evaluate 177Lu-PSMA-617 vs cabazitaxel for men with mCRPC after docetaxel. In this open label, phase II trial, 200 men were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax ≥ 20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥ 20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. Overall, 200 patients were randomized 1:1 to 177Lu-PSMA-617 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every 3 weeks for up to 10 cycles. Patients were stratified based on disease burden and prior anti-androgen therapy. The trial schema for TheraP is as follows: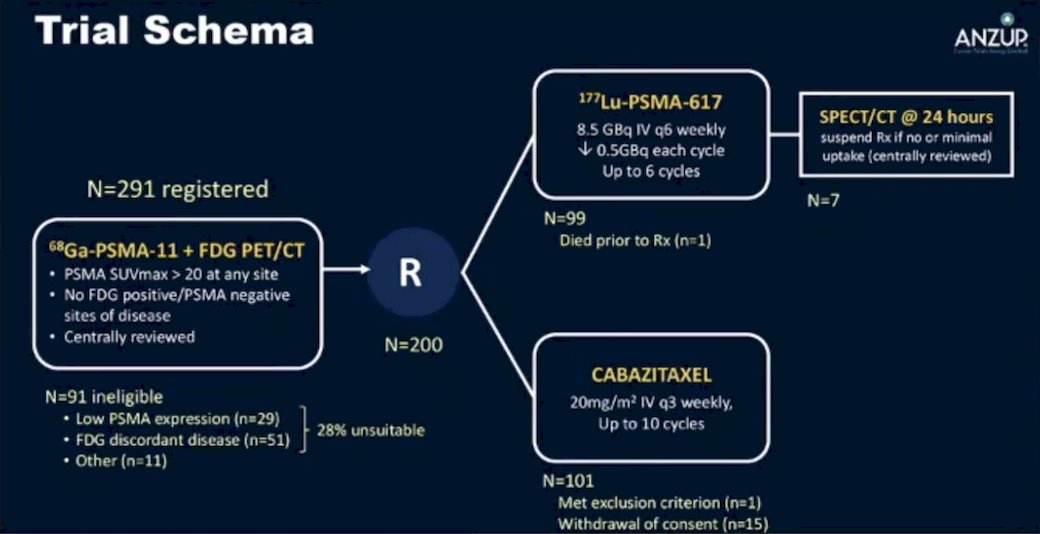
The primary endpoint of this study was a PSA decline of 50% (PSA50) and secondary endpoints included PSA-PFS and overall. After a median follow up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR 0.63, 95% CI 0.46 to 0.86) and a had a much higher PSA50 rate (66% vs 37%):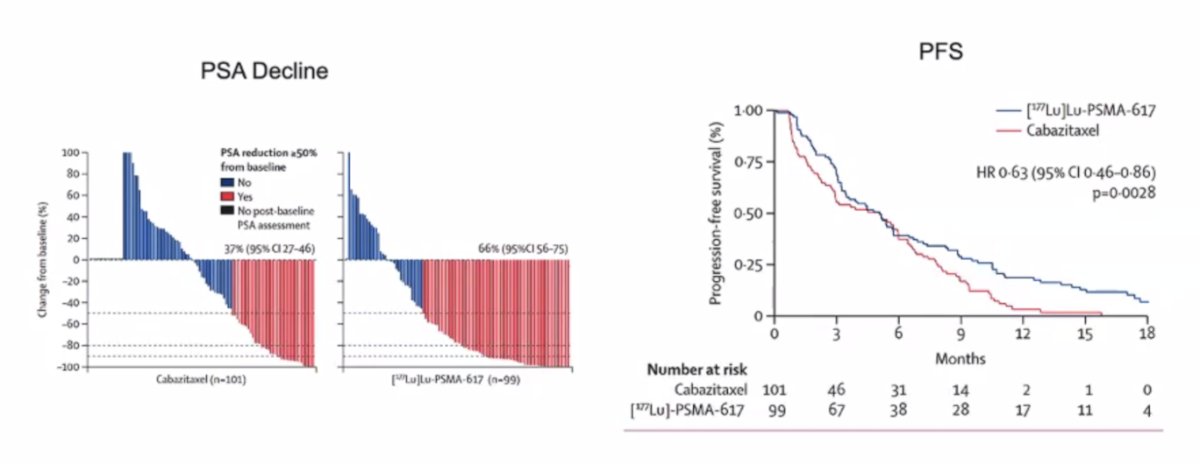
Similar to the VISION trial, nomograms have also been developed for PSMA and FDG-PET as predictive and prognostic biomarkers in patients from the TheraP trial.3 The HR for radiographic progression free survival for 177Lu-PSMA-617 versus cabazitaxel in men who had PSMA-PET SUVmean of at least 10 was 0.46 (95% CI 0.25–0.84), and was 0.85 (0.59–1.24) in men who had a PSMA-PET SUVmean of less than 10. Results were similar for PSA progression-free survival, with HRs for PSA progression-free survival of 0.45 (95% CI 0.25–0.80) for PSMA-PET SUVmean of at least 10 and 0.77 (0.53–1.12) for PSMA-PET SUVmean of less than 10: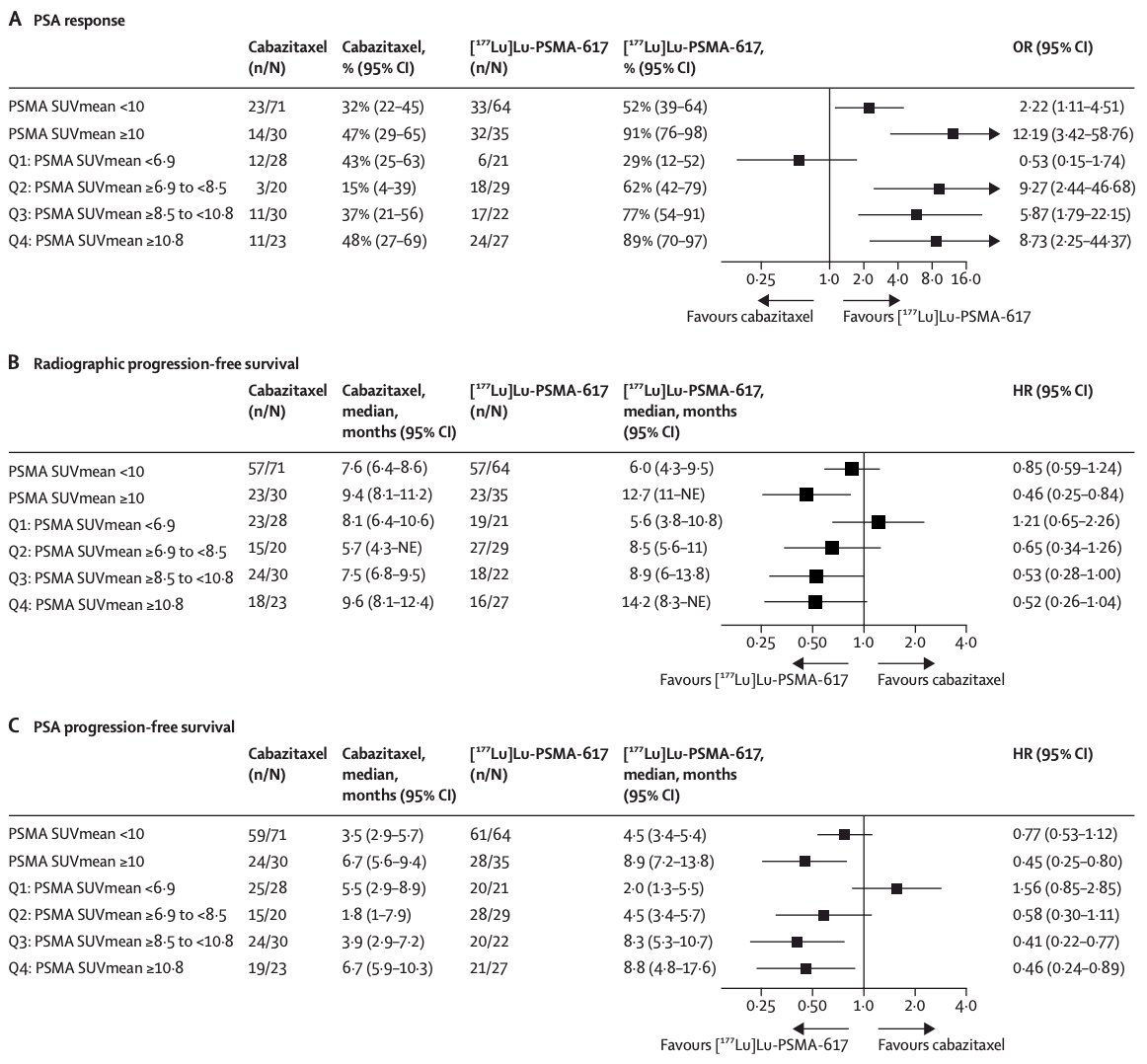
In work published in early 2024 in Lancet Oncology, Hofman and colleagues noted that after a median follow-up of 35.7 months (IQR 31.1 to 39.2), 77 (78%) participants had died in the 177Lu-PSMA-617 group and 70 (69%) participants had died in the cabazitaxel group.4 Overall survival was similar among those assigned to 177Lu-PSMA-617 versus those assigned to cabazitaxel (restricted mean survival time 19.1 months vs 19.6; difference -0.5 months; p = 0.77):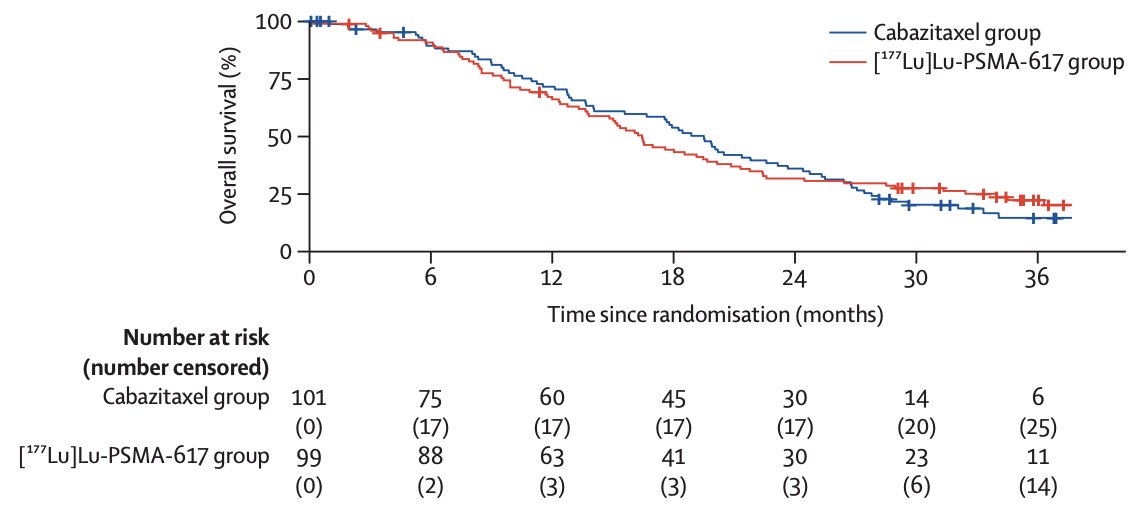
As such, these results support the use of 177Lu-PSMA-617 as an alternative to cabazitaxel for PSMA-positive metastatic castration-resistant prostate cancer progressing after docetaxel. Additionally, in this subsequent analysis of TheraP, PSMA PET and FDG PET were both prognostic for overall survival (top row: PSMA PET; bottom row: FDG PET):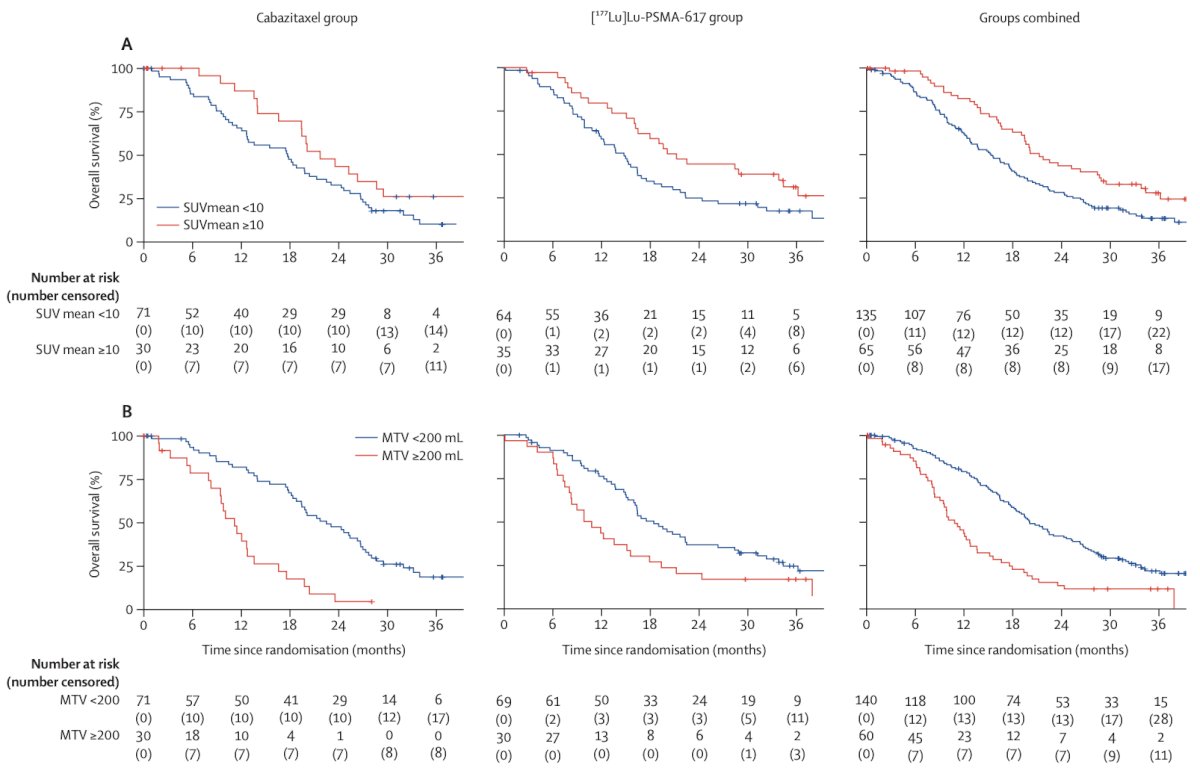
Dr. Morris concluded his presentation discussing the VISION and TheraP trials with the following brief tabular comparison of these two important clinical trials: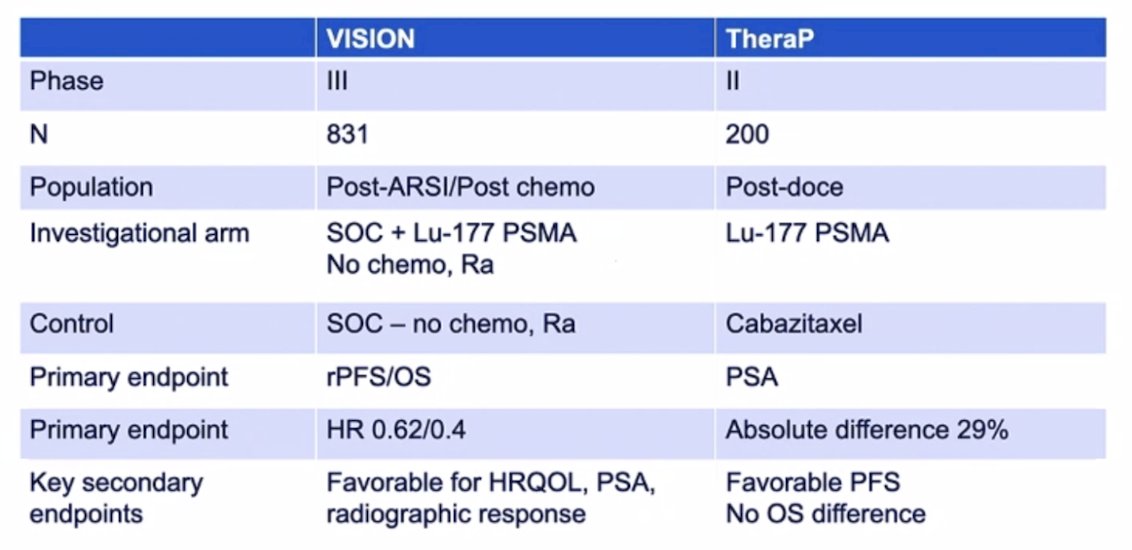
Presented by: Michael Morris, MD, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 PSMA Conference, San Francisco, CA, Thurs, Jan 18 – Fri, Jan 19, 2024.
Related content: Lutetium-177 PSMA Radioligand Therapy for Advanced Prostate Cancer: Reviewing the Pivotal VISION and TheraP Trials - Michael Morris
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Buteau JP, Martin AJ, Emmett L, et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): A biomarker analysis from a randomized, open-label, phase 2 trial. Lancet Oncol. 2022 Nov;23(11):1389-1397.
- Hofman MS, Emmett L, Sandhu S, et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): Secondary outcomes of a randomized, open-label, phase 2 trial. Lancet Oncol. 2024 Jan;25(1):99-107.


