(UroToday.com) The 2024 PSMA conference featured a presentation by Dr. Oliver Sartor discussing the role of radium-223 in the setting of PSMA PET. Dr. Sartor started by highlighting that based on the ALSYMPCA trial published in 2013,1 this was the first alpha emitter approved in all of medicine. Dr. Sartor shared that there are several shared elements between the ALSYMPCA trial and the VISION2 trial designs, including the disease state, “positive” and “negative” biomarkers, standard of care, and endpoints:

The ALSYMPCA trial was a phase 3, randomized, double-blind, placebo-controlled trial that randomized 921 patients in a 2:1 fashion to receive six injections of radium-223 (at a dose of 50 kBq per kilogram of body weight intravenously) or matching placebo. All patients received additional best standard of care. Of note, patients could have received prior docetaxel (57% of included patients). The primary endpoint was overall survival and patients receiving radium-223 had significantly improved median overall survival (14.9 versus 11.3 months; HR 0.70, 95% CI 0.58 to 0.83):
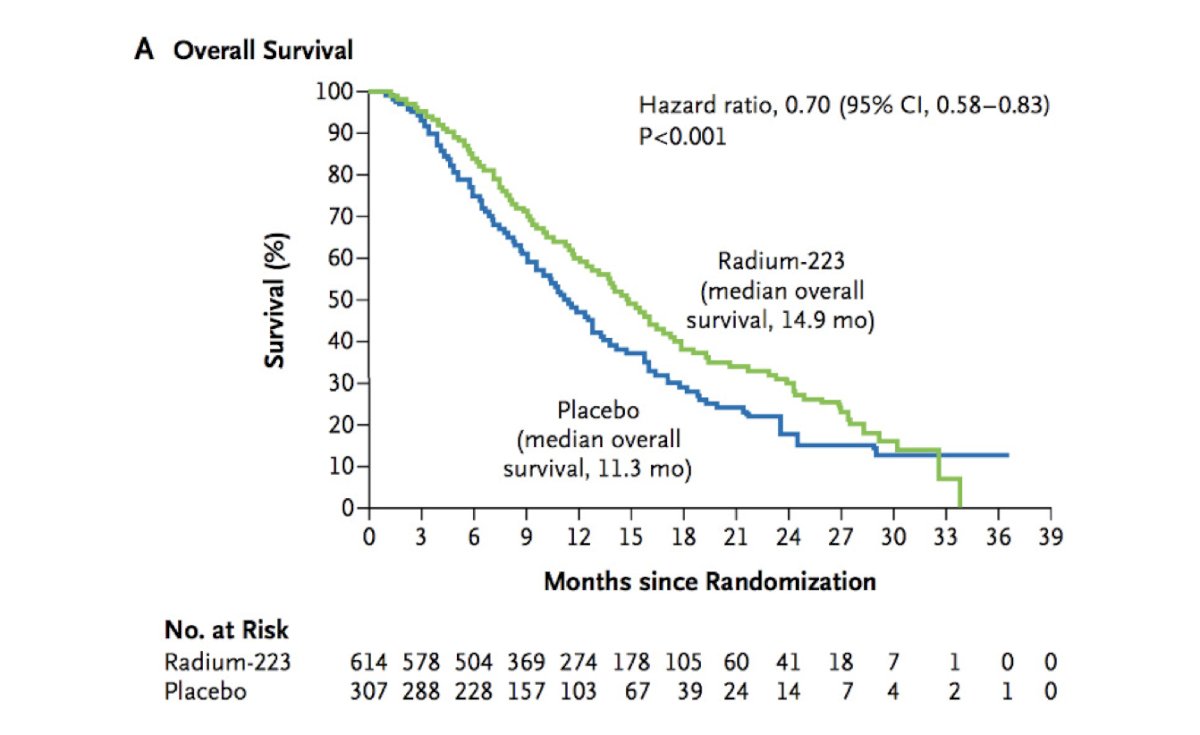
Use of radium-223 was further associated with significantly prolonged time to the first symptomatic skeletal event (15.6 versus 9.8 months, p<0.001), time to increase in the total alkaline phosphatase level (HR 0.17, p < 0.001), and time to increase in PSA level (HR 0.64, p < 0.001). Dr. Sartor highlighted that the standard therapies available (delineated by castrate sensitive vs metastatic castrate resistant disease states) at the time of ALSYMPCA accrual in 2008 were quite scant and as follows:

In contrast, the standard of care therapies available today are as follows:
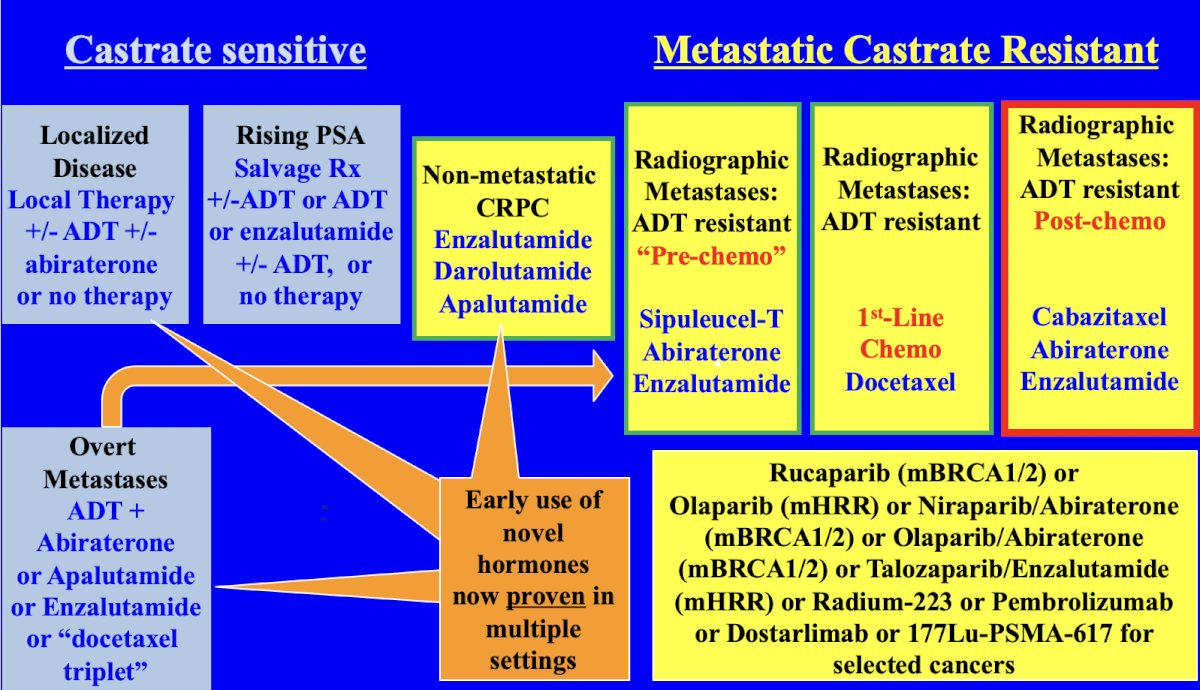
Dr. Sartor notes that there are several problems with radium-223. First, how do we interpret the positive ALSYMPCA trial in the context of an immensely changed therapeutic landscape? As noted above, the treatment landscape in 2008 is drastically different than in 2024. Second, radium-223 targets activated bone stromal lesions but does not target tumors in soft tissue. As such, better patient selection is needed for radium-223. Positive selection criteria includes: bone scans, and possibly F-18 NaF PET uptake, which binds avidly and quantitatively to bone stroma. Negative selection criteria includes: exclusion of PSMA PET positive visceral and soft tissue lesions, which have greater sensitivity compared to CT or MRI scans. Work from Ahmadzadehfar and colleagues3 has previously looked at 68Ga-PSMA-11 PET as a gatekeeper for the treatment of metastatic prostate cancer with radium-223. As can be seen in the following PSA waterfall plots, patient selection with bone scan and PSMA PET/CT is a move in the right direction compared to with bone scan and CT scan only:

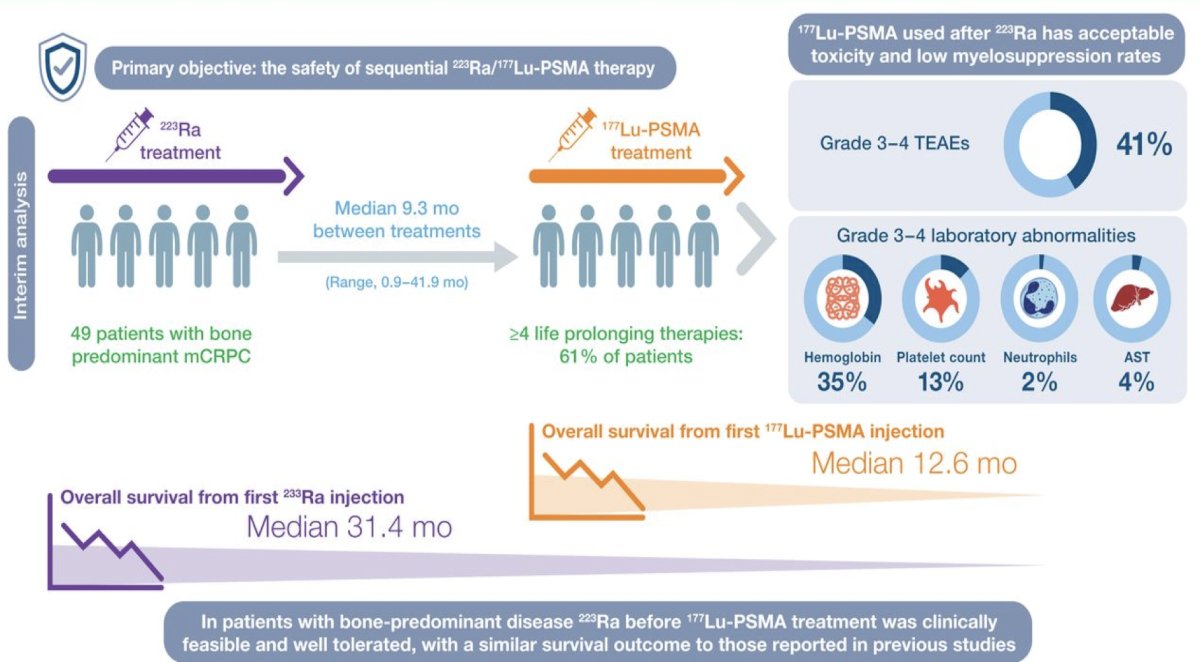
Additionally, Dr. Sartor notes that both PSMA PET and diffusion weighted MRI (ADC phase) provide better response monitoring after radium-223 treatment. The interim analysis of the RALU study,4 published in 2023, showed that the median time between treatment with radium-223 and 177Lu-PSMA therapy was 9.3 months (range: 0.9-41.9), with an acceptable toxicity given the grade 3-4 treatment emergent adverse event rate was 41%. The median overall survival from the first radium-223 injection was 31.4 months and the overall survival from the first 177Lu-PSMA injection was 12.6 months. Thus, in patients with bone-predominant disease, radium-223 before 177Lu-PSMA treatment was clinically feasible and well tolerated, with a similar survival outcome to those reported in previous studies:
Dr. Sartor then discussed new phase 3 studies utilizing radium-223. The first discussion was EORTC GUCG 1333 (PEACE III), which is randomizing men with asymptomatic bone predominant mCRPC and no known visceral metastasis 1:1 to enzalutamide + radium-223 versus enzalutamide alone. The primary endpoint for this trial is radiographic progression free survival:

Importantly, Dr. Sartor noted that based on work presented by Bertrand Tombal at ASCO 2019, the interim safety analysis of the PEACE III trial showed a decreased fracture rate by mandating bone protecting agents, thus PEACE III was amended to require bone protective agents:

Small numbers beyond month 20
The second new phase 3 trial incorporating radium-223 is the DORA trial, which is a phase III trial of docetaxel versus docetaxel and radium-223 for metastatic castration-resistant prostate cancer.
Finally, there are two earlier phase studies using radium-223 that Dr. Sartor mentioned, including:
- AlphaBet: The combination of radium-223 and 177Lu-PSMA-I&T in men with metastatic castration-resistant prostate cancer
- BAT-RAD: Bipolar androgen therapy (BAT) and radium-223 in metastatic castration-resistant prostate cancer
Dr. Sartor concluded his presentation discussing the role of radium-223 in the setting of PSMA PET with the following take-home points:
- Radium-223 suffers from lack of new data with the only positive phase 3 trial being conducted in a different era (which started in 2008)
- Various combinations of radium-223 are in current phase III clinical trials – PEACE III and DORA
- There are some interesting combination studies underway
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 PSMA Conference, San Francisco, CA, Thurs, Jan 18 – Fri, Jan 19, 2024.
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Ahmadzadehfar H, Azgomi K, Hauser S, et al. 68Ga-PSMA-11 PET as a Gatekeeper for the treatment of metastatic prostate cancer with 223Ra: Proof of Concept. J Nucl Med. 2017 Mar;58(3):438-444.
- Rahbar K, Essler M, Eiber M, et al. Safety and Effectiveness of Lutetium-177-Prostate Specific Membrane Antigen (177Lu-PSMA) Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Previously Treated with Radium-223 (223Ra): The RALU Study. J Nucl Med. 2023 Dec 1;64(12):1925-1931.


