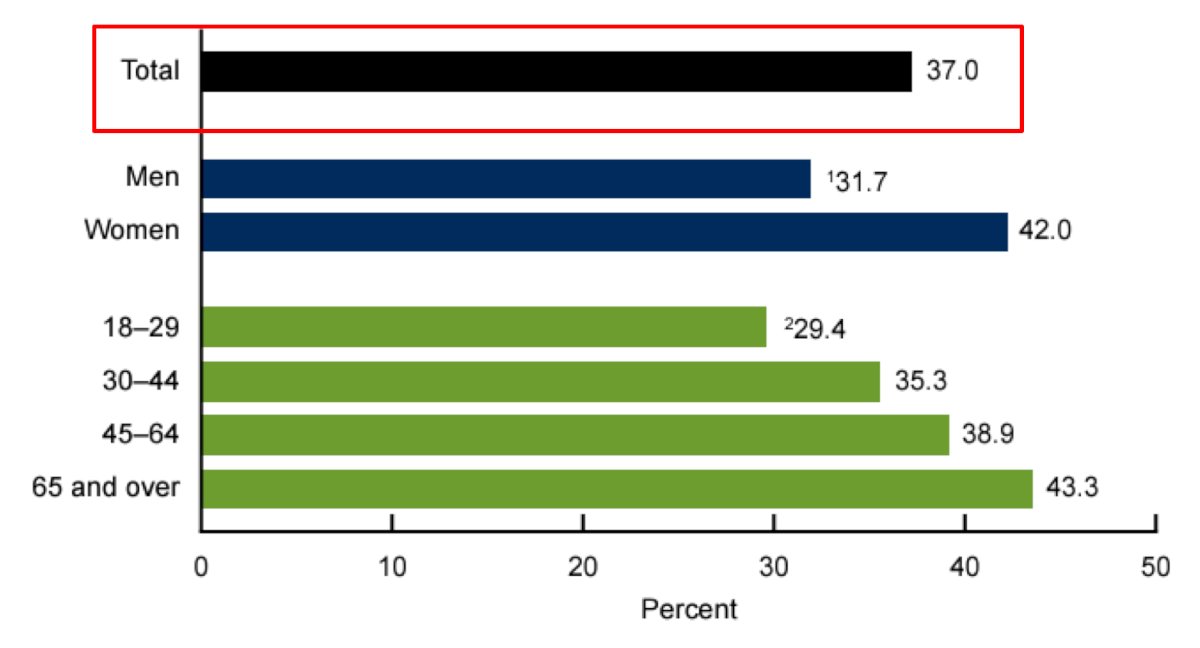
(UroToday.com) The 2024 Southeastern Section of the AUA (SESAUA) annual meeting featured a State of the Art Lecture by Dr. Timothy Lyon discussing in-home therapy for bladder cancer. Dr. Lyon started his presentation by emphasizing that the COVID-19 pandemic altered the delivery of and access to healthcare across the United States. In April 2021, the U.S. Department of Health and Human Services noted that emergency department visits were down 40%, in person visits were postponed or changed to telehealth visits, and elective procedures were routinely postponed. Indeed, among US adults in 2021, 37.0% had used telemedicine in the past 12 months, which was more common in women and in those 65 years of age or older:
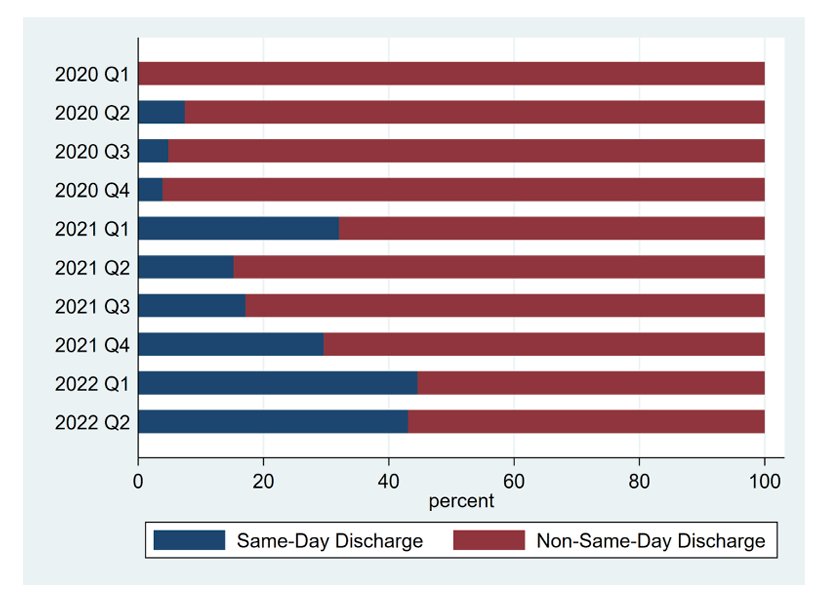
With regards to urology, during and immediately after the pandemic, there was an increase in the institutional trends of same day discharge for robotic assisted laparoscopic radical prostatectomies.1 The proportion of same day discharge increased from 4.4% in the fourth quarter of 2020 to 45% in the second quarter of 2022 (p < 0.01):
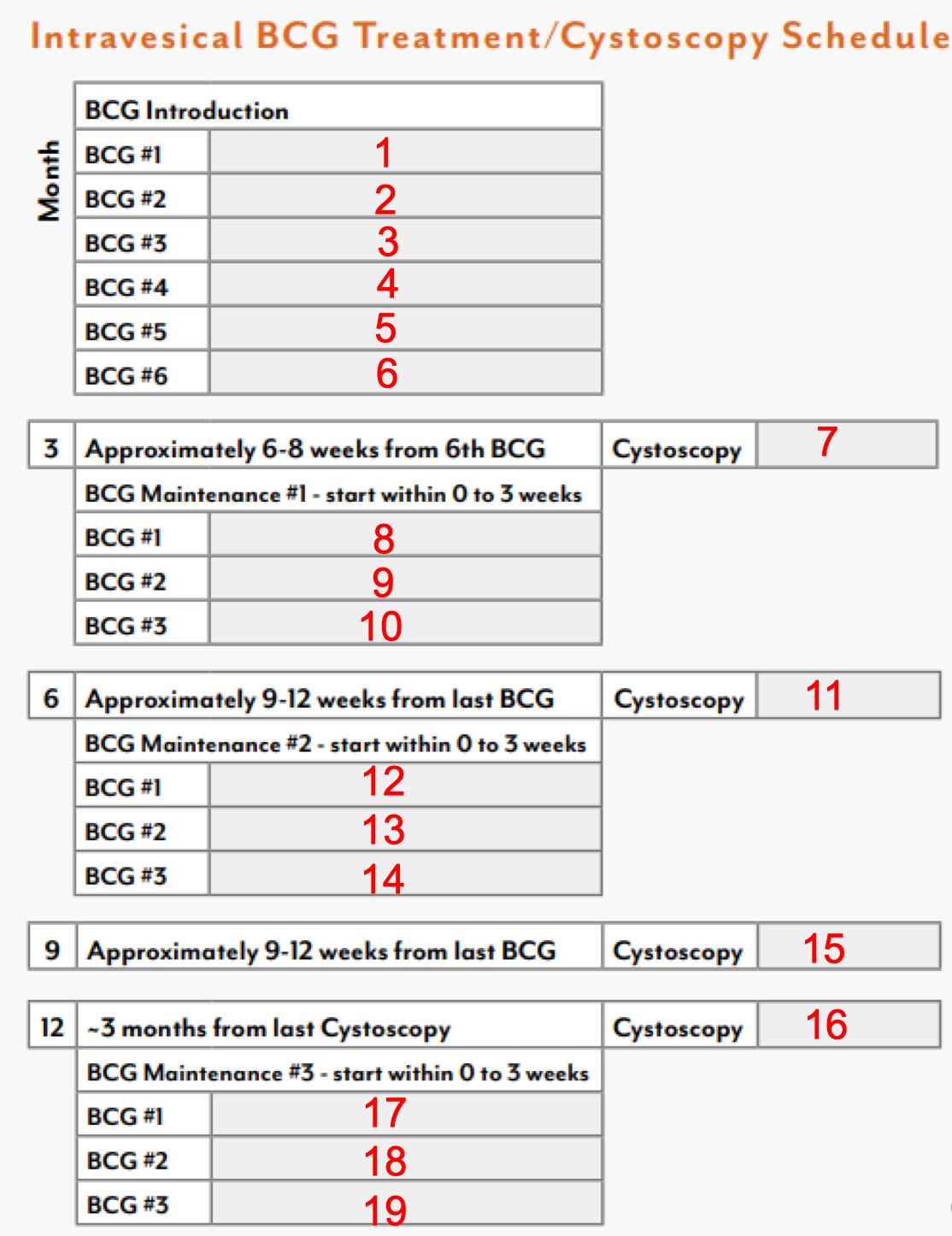
Dr. Lyon highlighted that bladder cancer represents 4.2% of all new cancer cases in the United States, with an estimated 82,290 new cases and 16,710 mortalities. For non-muscle invasive bladder cancer, BCG intravesical therapy has been a mainstay of therapy for nearly 50 years, however, a full BCG induction protocol and three rounds of maintenance is arduous, with ~19 in person visits for the patient:
A study assessing two systematic literature reviews was previously conducted to further assess the current evidence on BCG use in NMIBC and the humanistic and economic burden of disease.2 This analysis showed that among 23 studies reporting HRQoL and symptoms in NMIBC, HRQoL at diagnosis was comparable with population norms but worsened considerably 2 years following diagnosis:
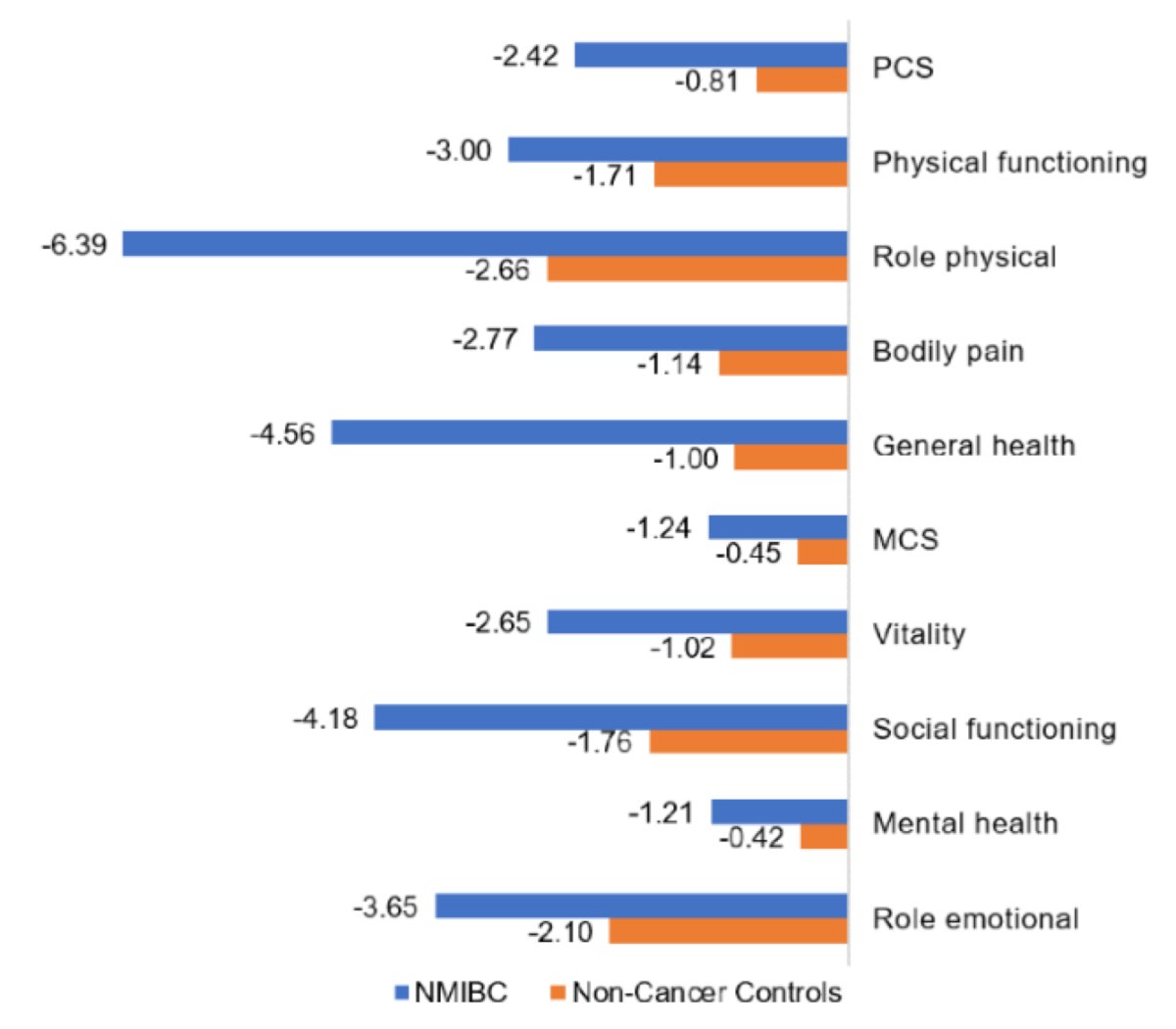
Moreover, maintenance therapy with intravesical BCG was associated with reduced HRQoL, and treatment-related adverse events resembled typical NMIBC symptoms. Twenty-two studies reported decreasing BCG compliance over time. Dr. Lyon notes that generally intravesical therapy discontinuation rates have been higher than expected from adverse events alone. In the SWOG maintenance trial, only 16% completed 3 years of maintenance therapy, and in a Spanish maintenance randomized trial, 34.5% completed 3 years of maintenance therapy, with 10% reporting stopping due to adverse events or toxicity.
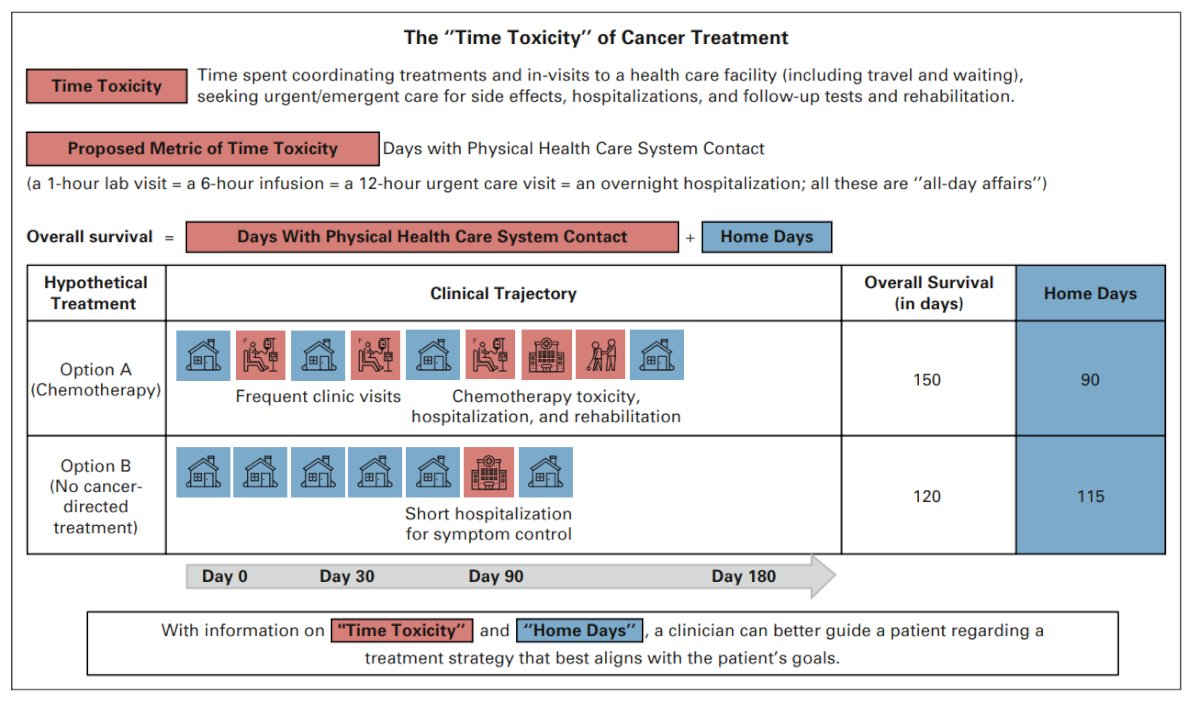
In their 2022 Journal of Clinical Oncology article, Gupta and colleagues3 highlighted the time toxicity of cancer treatment. Time toxicity has been defined as time spent in coordinating care and in frequent visits to a health care facility (including travel and wait times), seeking urgent/emergent care for side effects, hospitalization, and follow-up tests. By way of example, a patient may view treatments differently if they knew that, on average, three of their remaining estimated nine months alive would be spent away from home (for infusions, in the hospital, etc) if they pursued treatment option A, but that all of their estimated remaining seven months would be spent at home if they pursued option B:3
With regards to in-home intravesical therapy, this has the potential to (i) reduce patient workload, (ii) increase access, (iii) improve number of home days, and (iv) improve the patient experience. In 2023, Dr. Lyon and colleagues performed a narrative review assessing the literature for in-home intravesical therapy for NMIBC, finding only 2 studies.4 The concept of in-home intravesical therapy started in 1991 with BCG being reconstituted in the home, placing the Foley catheter, and clamping for 2 hours. In 2006, a pilot program in Newcastle, UK, assessed an in-home approach with 324 BCG instillations and 90 chemotherapy instillations; patients reported “extremely high” satisfaction with this approach.
Recently, Dr. Lyon’s group looked at patient reported treatment burden and attitudes towards in-home intravesical therapy.5 This study was a cross-sectional survey of the Bladder Cancer Advocacy Network (BCAN) Patient Survey Network. Among 233 patients responding to the survey, 66% had received greater than 12 bladder instillations. A travel time >30 minutes to an intravesical treatment facility was reported by 55% of patients, and 33% reported personal out-of-pocket costs greater than $25 associated with each trip:
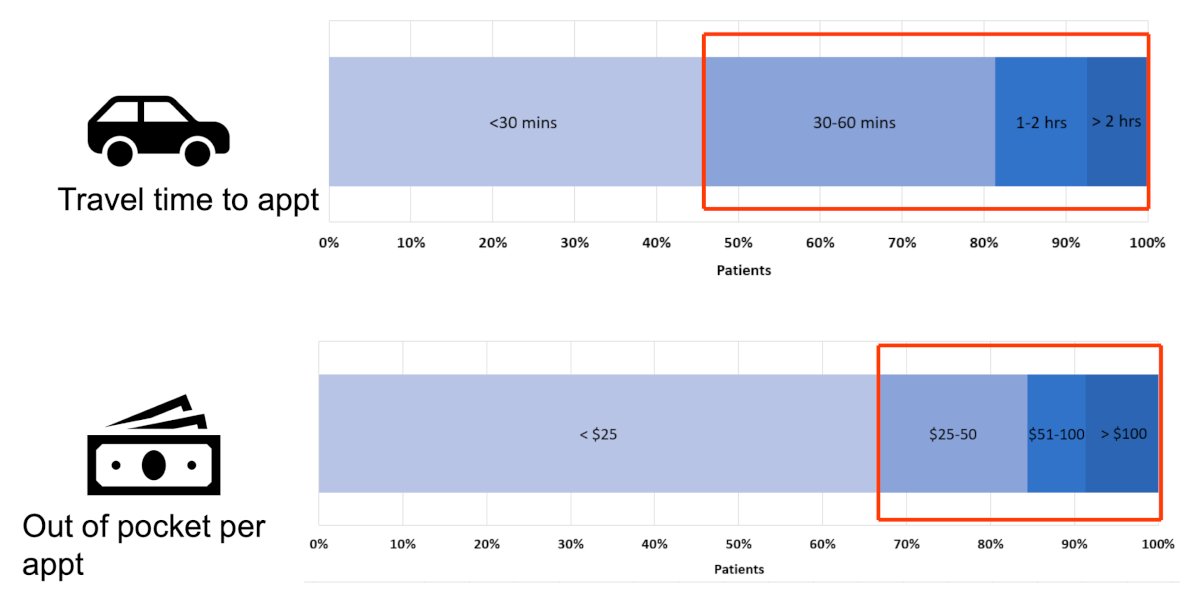
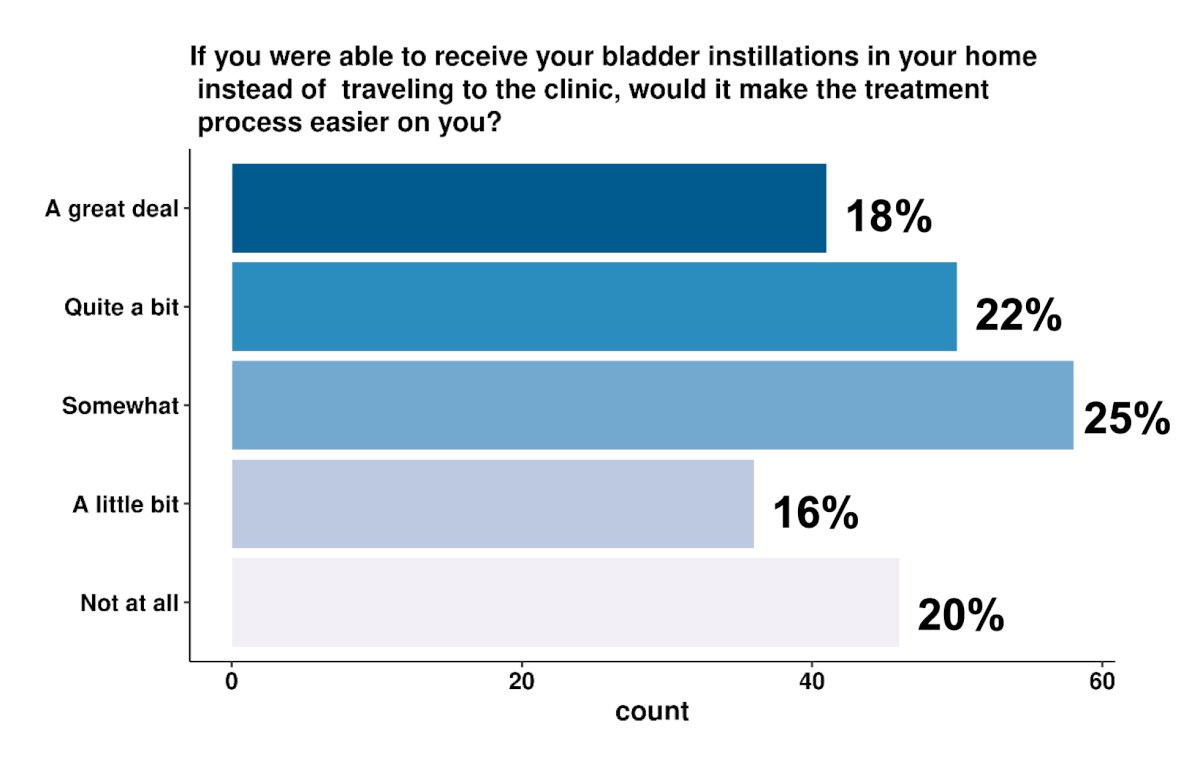
Missing work for intravesical installations was reported by 36% of patients, and of those who did, the majority (70%) missed at least half a day of work (4 or more hours). Fifty-six percent of patients brought caregivers to their appointments. More than half of patients (56%) reported spending more than 2 hours on each intravesical instillation, with 18% spending more than 4 hours. Moreover, 72% reported openness to receiving in-home intravesical instillations and 54% answered that in-home instillations would make the treatment process less disruptive to their lives:
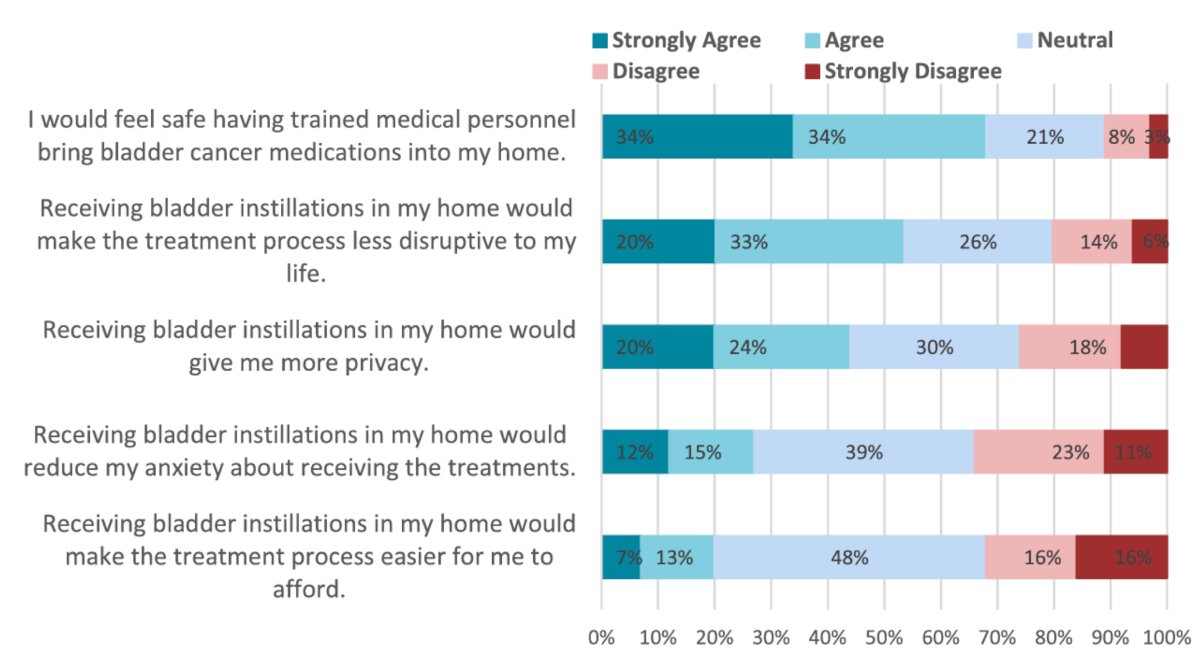
In general, patients did not view the in-home treatments as easier to afford or reduce anxiety about treatments. However, patients did feel that in-home treatments would improve privacy, make the treatment process less disruptive to their lives and the overwhelming majority would feel safe. Among those surveyed, 68% reported that they would feel safe with medical personnel bringing bladder cancer medications into their home, 54% answered that in-home instillations would make the treatment process less disruptive to their lives, and 27% reported that in-home therapy would reduce their anxiety around intravesical instillations:
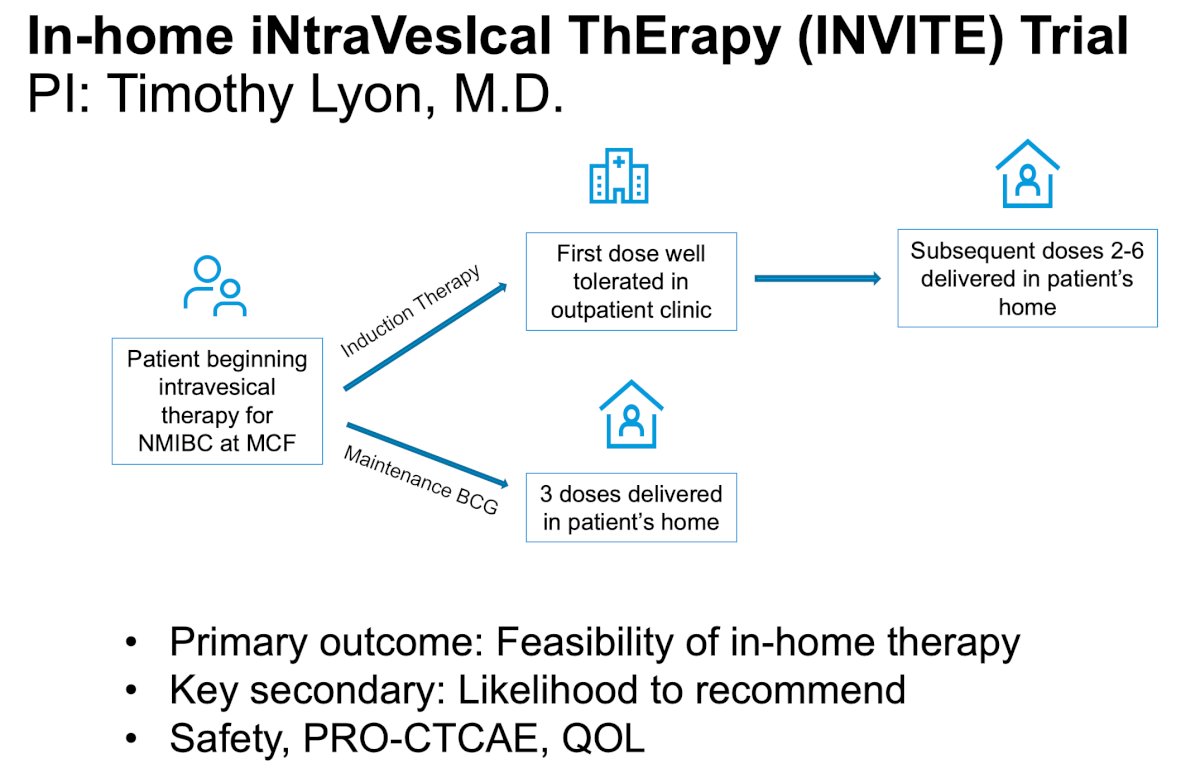
Dr. Lyon is the PI of the In-home iNtraVesIcal ThErapy (INVITE) trial which is assessing the feasibility of in-home therapy as a primary outcome. The trial design for INVITE is as follows:
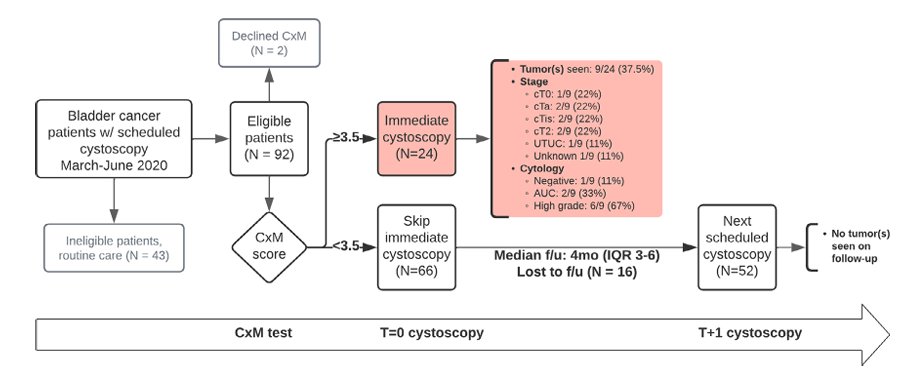
With regards to bladder cancer surveillance, Dr. Lyon highlighted Cxbladder monitoring to potentially reduce the frequency of cystoscopy in patients with bladder cancer. Li and colleagues previously reported outcomes from a prospective multi-institutional study of Cxbladder monitor to reduce cystoscopies during the COVID-19 pandemic.6 Among 92 patients, 9 of 24 (37.5%) Cxbladder monitor-positive patients had 1 T0, 2 Ta, 2 Tis, 2 T2, and 1 upper tract urothelial carcinoma on immediate cystoscopy and subsequent evaluation. 66 Cxbladder monitor-negative patients skipped cystoscopy, of which none had findings on follow-up cystoscopy requiring biopsy:
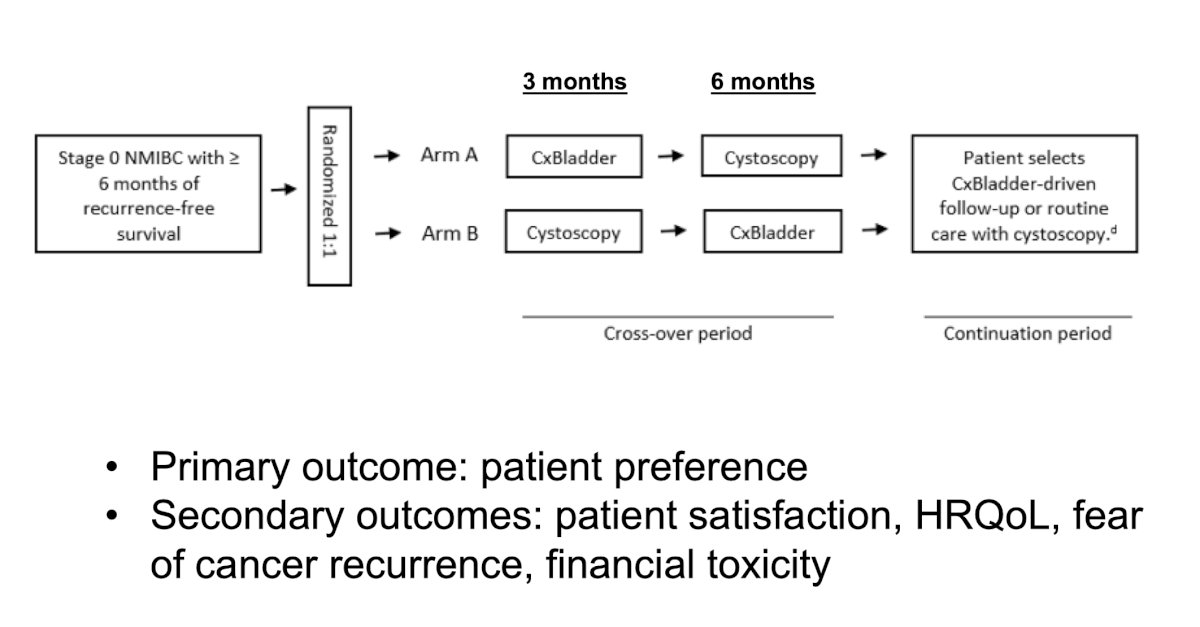
Dr. Mark Tyson is leading a randomized cross-over study of Cxbladder monitor versus cystoscopy among stage 0 NMIBC patients with >= 6 months of recurrence-free survival, with the following trial design:
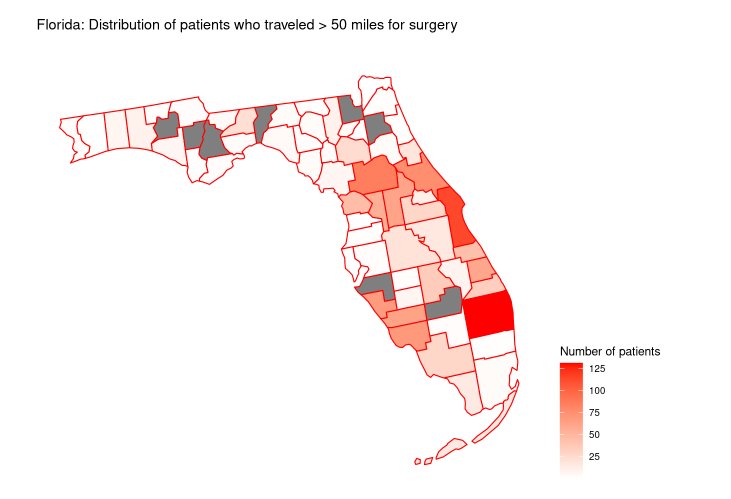
For post-operative considerations, urinary tract infection is a leading cause of morbidity and readmission after radical cystectomy: 30% rate at 90 days in the RAZOR trial,7 and 36% rate at 90 days in the large USC cohort.8 Dr. Lyon also notes that the travel distance for radical cystectomy in Florida can be arduous: among 4,209 patients, 1,271 (30%) traveled> 50 miles for surgery:
Advanced care at home after surgery seems plausible. Work from Mayo Clinic Wisconsin and Mayo Clinic Florida suggest the following potential algorithm:9

Overall, 56 patients were admitted to advanced care at home from 2020-2022 for a GU diagnosis or after GU surgery. The majority of admissions were for infectious reasons (98%): pyelonephritis (91%), epididymitis (4%), cellulitis (2%), and diverticulitis (2%). The median length of stay was 3 days (range: 1-31 days), and 216 inpatient hospital days were saved.
Two studies are assessing in-home care for advanced disease. The first is at-home cancer directed therapy versus in clinic for advanced care, with patients receiving systemic chemotherapy eligible for the trial. Patients receive their first two cycles in the clinic and then are randomized to: (a) in home therapy for 24 weeks vs (b) in clinic therapy for 8 weeks then home therapy for 16 weeks. The primary outcome for this trial is patient-reported rate of in-home therapy, and secondary outcomes include patient preference, safety, and health related quality of life. The second trial is a comparison of in-home versus in-clinic subcutaneous nivolumab. This is a single arm study of patients receiving single agent nivolumab, including 8 weeks in office followed by 8 weeks at home. The primary outcome is a change in patient-reported rate of CARE program after 8 weeks in clinic compared to 8 weeks at home. The secondary endpoints include patient preference, safety, health related quality of life, ER visits, and hospitalizations.
Dr. Lyon concluded his presentation by discussing in-home therapy for bladder cancer with the following summary statements:
- In-home therapy for bladder cancer is coming across the disease spectrum
- In-home intravesical therapy has the potential to decrease treatment burden and improve treatment satisfaction
- We owe it to our patients to develop innovative methods of care delivery to decrease time toxicity and improve patient experience of bladder cancer treatment
Presented by: Timothy Lyon, MD, Mayo Clinic, Jacksonville, FL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Southeastern Section of the American Urological Association (SESAUA) Annual Meeting, Austin, TX, Wed, Mar 20 – Sat, Mar 23, 2024.
References:
- Labban M, Frego N, Qian ZJ, et al. Institutional trends and safety profile of same-day discharge for robot-assisted laparoscopic radical prostatectomy: A retrospective analysis. Urol Oncol. 2023 Aug;41(8):354e.19-354.e26.
- Lee LJ, Kwon CS, Forsythe A, et al. Humanistic and Economic Burden of Non-Muscle Invasive Bladder Cancer: Results of Two Systematic Literature Reviews. Clinicoecon Outcomes Res. 2020 Nov 23;12:693-709.
- Gupta A, Eisenhauer EA, Booth CM. The Time Toxicity of Cancer Treatment. J Clin Oncol. 2022 May 20;40(15):1611-1615.
- Lyon TD, Boorjian SA, Tyson MD. In-home Intravesical Therapy: The Future of Nonmuscle-invasive Bladder Cancer Care Delivery? J Urol. 2023 Apr;209(4):656-658.
- Myers A, Ristau B, Mossanen M, et al. Patient reported treatment burden and attitudes towards in-home intravesical therapy among patients with bladder cancer. Urol Oncol. 2024 Feb;42(2):29.e17-29.e22.
- Li KD, Chu CE, Patel M, et al. Cxbladder Monitor testing to reduce cystoscopy frequency in patients with bladder cancer. Urol Oncol. 2023 Jul;41(7):326.e1-326.e8.
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomized, phase 3, non-inferiority trial. Lancet 2018 Jun 23;391(10139):2525-2536.
- Ghoreifi A, Van Horn CM, Xu W, et al. Urinary tract infections following radical cystectomy with enhanced recover protocol: A prospective study. Urol Oncol. 2020 Mar;38(3)75.e9-75.e14.
- Paulson MR, Torres-Guzman RA, Avila FR, et al. Severity of illness and risk of mortality in Mayo Clinic’s virtual hybrid advanced care at home program: A retrospective cohort study. BMC Health Serv Res. 2023 Mar 27;23(1):287.


