(UroToday.com) The 2024 Southeastern Section of the AUA (SESAUA) annual meeting featured an AUA Course Lecture and a presentation by Dr. Marc Bjurlin discussing current approaches and future opportunities for integrating molecular imaging into urologic oncology clinical practice. As part of this AUA course, Dr. Bjurlin noted that, in addition to himself, there were several faculty involved including the following:
- Spencer Behr, MD: Associate Professor of Clinical Radiology at University of California, San Francisco
- Michael A. Gorin, MD: Faculty in the Milton and Carroll Petrie Department of Urology at the Icahn School of Medicine at Mount Sinai, NY
- Tracy L. Rose, MD, MPH: Associate Professor in Medical Oncology at the University of North Carolina Lineberger Comprehensive Cancer Center
Dr. Bjurlin highlighted that regardless if the patient has kidney, bladder, prostate, or testis cancer, the following figure highlights a possible diagnostic algorithm:
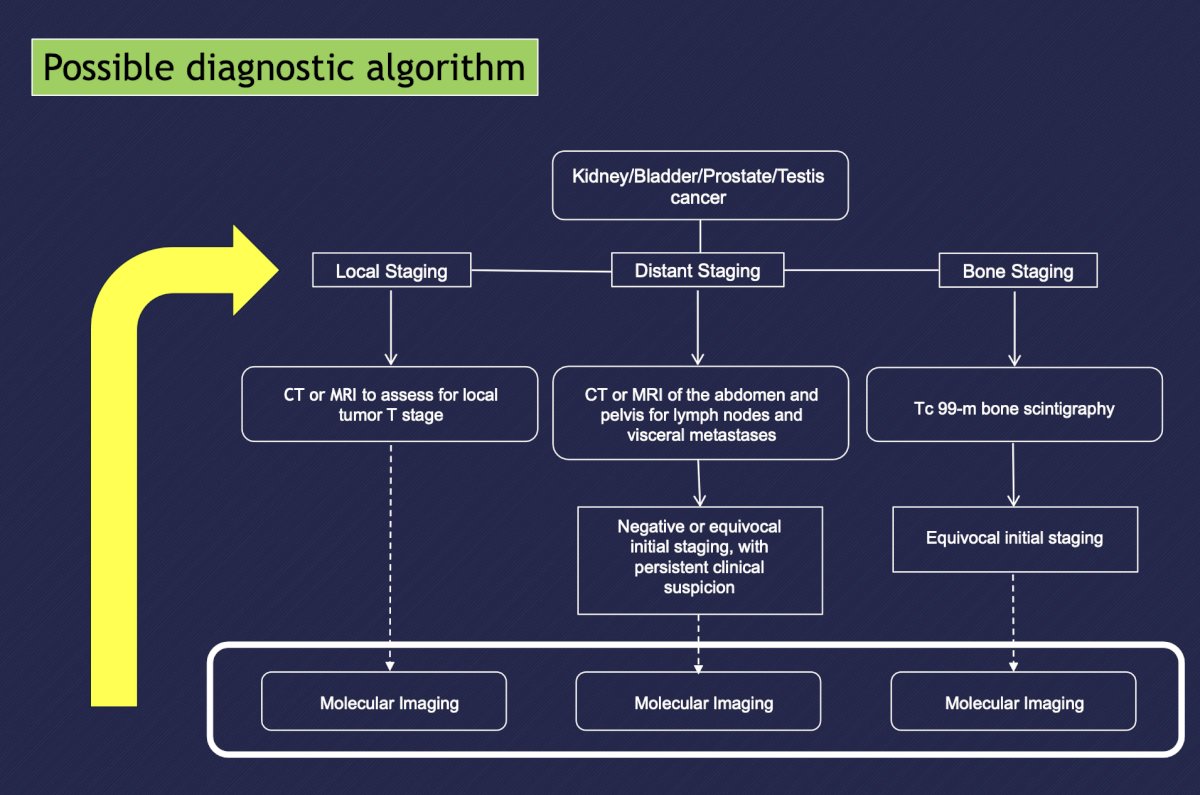
Dr. Bjurlin started by discussing molecular imaging in prostate cancer, with slides courtesy of Dr. Behr. Currently, there are several radiopharmaceuticals studied in prostate cancer:
- Choline (11C, 18F, 18F-fluoroethylcholine)
- Acetate (11C, 18F)
- 18F-FDG
- FACBC (18F-flucicolovine)
- 11C-serotonin
- 11C-methionine
- 18F-FDHT
- PSMA (68Ga, 18F, 111In, 177Lu)
- Bombesin (68Ga, 18F, Tc99m, 177Lu)
- NaF, 99mTc-MDP, Ra-223
Radiotracers 99mTc-labeled diphosphonates (MDP and HDP) and 18F NaF have a mechanism of incorporating in the bone matrix, thus images are secondary to osteoblastic activity and not tumor burden. FDA approval is for metastatic bone disease and response to therapy. F18-NaF PET/CT for staging is more accurate and sensitive than 99mTc-labeled diphosphonates, has higher specificity with CT, but does not allow assessment of soft tissues. The following figure highlights a historical perspective of staging imaging in the context of ideal minimum PSA levels:
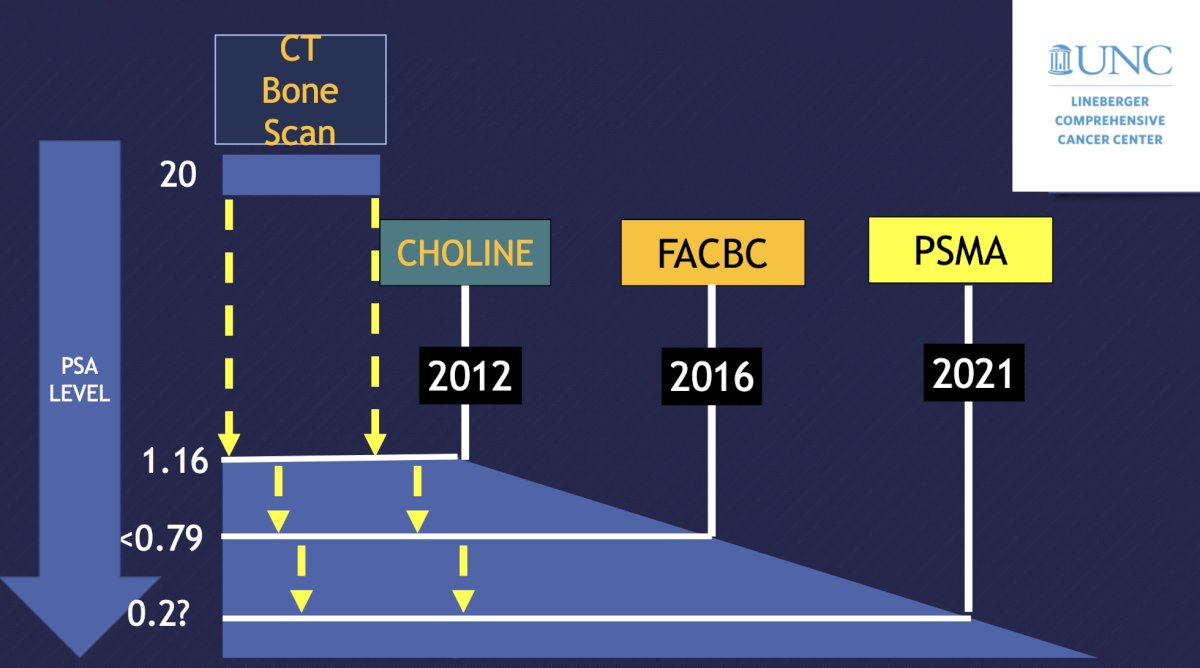
Dr. Bjurlin notes that PSMA has a normal biodistribution in the lacrimal glands, salivary glands, liver, spleen, bowel, kidneys, ureters, and urinary bladder. The clinical indications for PSMA PET/CT are delineated by each guideline:
- AUA
- Initial staging: high risk for metastatic disease with negative conventional imaging
- Recurrence: PSA recurrence after failure of local therapy or in the setting of negative conventional imaging
- NCCN
- Initial staging: equivocal results on initial bone imagine or soft tissue imaging
- Recurrence: preferred for bone and soft tissue (full body) imaging
- SNMMI Appropriate Use Criteria
- Newly diagnosed unfavorable intermediate-, high-, or very-high-risk prostate cancer
- Recurrence
- Non-metastatic castrate resistance prostate cancer (M0) on conventional imaging
With regards to imaging before a prostatectomy, an MRI provides T-staging and an accurate local assessment of disease involvement (extracapsular extension, seminal vesicle invasion, etc). PSMA PET/CT improves the detection of the primary, as well as nodal and metastatic disease, and may even outperform MRI. A 2020 meta-analysis of studies in high-risk pre-prostatectomy patients noted that PSMA PET had a sensitivity of 23-100%, specificity of 67-100%, PPV of 20-100%, and NPV of 41-100%, also suggesting that PSMA PET is superior to anatomical imaging (CT and MRI):
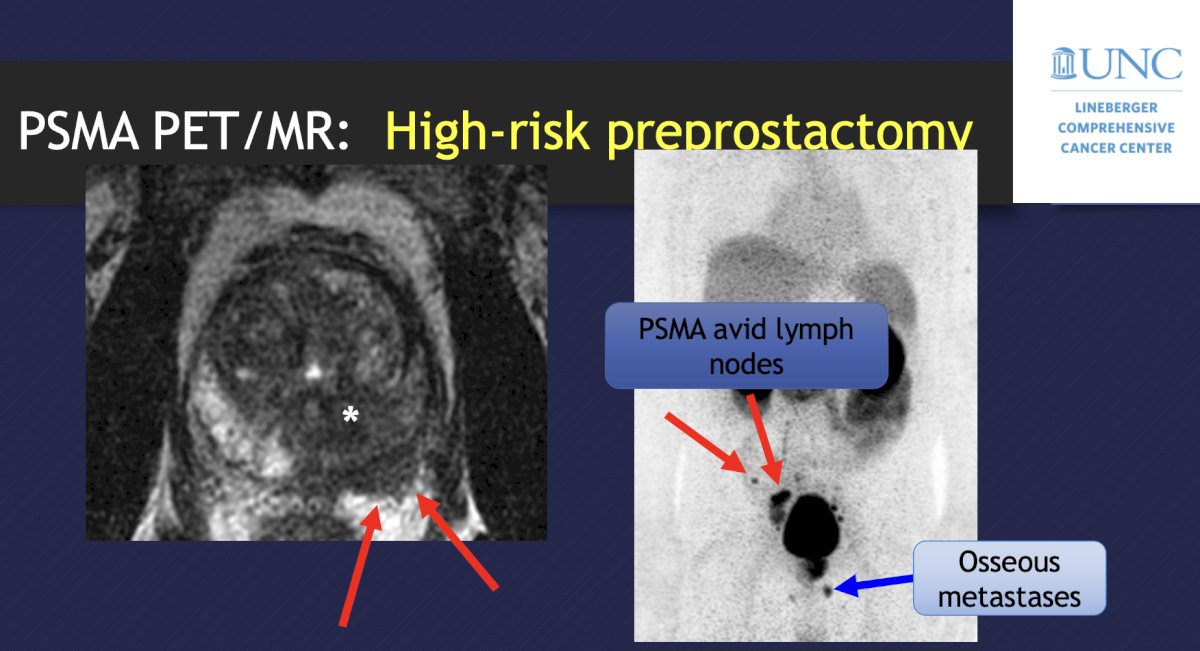
In the biochemical recurrent setting, PSMA PET should be considered post-prostatectomy PSA > 0.2 ng/mL and for a post-radiotherapy rise of serum PSA 2 ng/mL or more above nadir. As follows is an example of a 68-year-old with a PSA of 2.5 ng/mL after radical prostatectomy:

Several studies have looked at the impact of PSMA PET/CT on patient care, including a 2018 meta-analysis that found that PSMA PET changes management in ~54% of cases.1 There were increases in the utilization of radiotherapy (56% 61%), surgery (1% 7%), focal therapy (1% 2%), and multimodal treatment (2% 6%). However, there were also decreases in systemic treatment (26% 12%) and no treatment (14% 11%). Dr. Bjurlin’s key takeaways from this section of his talk regarding molecular imaging and prostate cancer were as follows:
- There has been incredible change in molecular imaging in prostate cancer
- PSMA PET is exceptionally sensitive with a significant impact on patient care, even in situations of a low PSA
- PSMA uptake is not as specific as the name would imply, and if it does not look like typical prostate cancer metastasis, we must consider other etiologies (thyroid, RCC, HCC, etc). Furthermore, we must be careful with solitary bone lesions (false positives)
- Conversely, not all prostate cancers are PSMA avid
Dr. Bjurlin then discussed the histologic characterization of the indeterminate renal mass with molecular imaging, with slides courtesy of Dr. Gorin. The histology of indeterminate small renal masses is vast, with ~15% of these entities being benign:

As such, can we use molecular imaging to differentiate between the various renal tumor histologies? Two types of molecular imaging modalities include 99mTc-sestabmibi and G250/girentuximab/TLX25-CDx (targeting carbonic anhydrase IX):
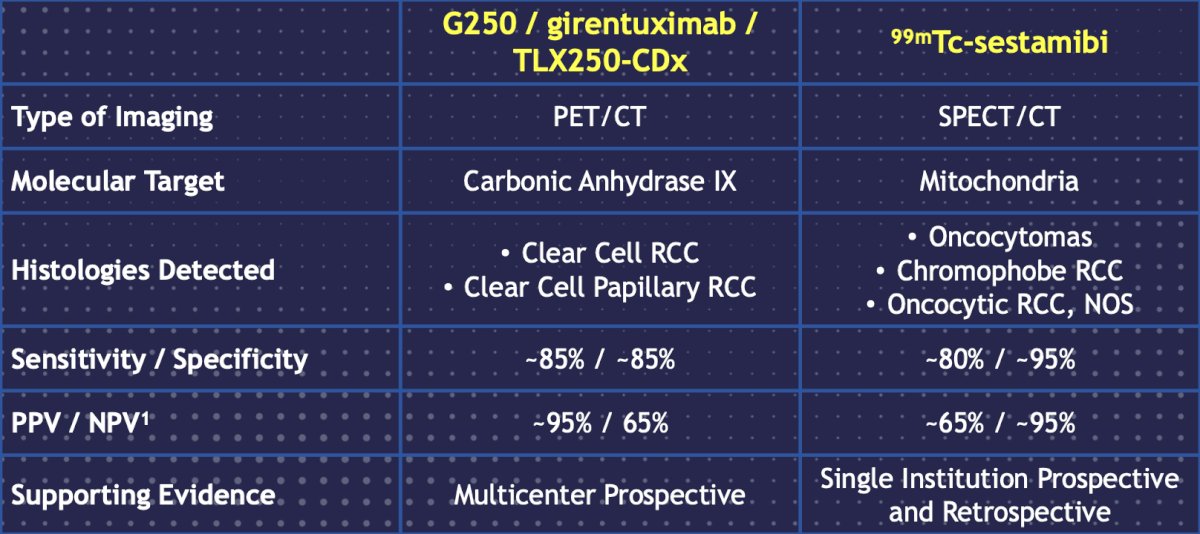
Dr. Bjurlin discussed the results of the ZIRCON trial, which was initially presented at ASCO GU 2023. ZIRCON was an open-label, multicenter clinical trial. Patients with an indeterminate renal masses (≤ 7 cm; tumor stage cT1) who were scheduled for partial nephrectomy within 90 days from planned TLX250-CDx administration were eligible. Enrolled patients received a single dose of TLX250-CDx IV (37 MBq ± 10%; 10 mg girentuximab) on Day 0 and underwent PET/CT imaging on Day 5 (± 2 days) prior to surgery:
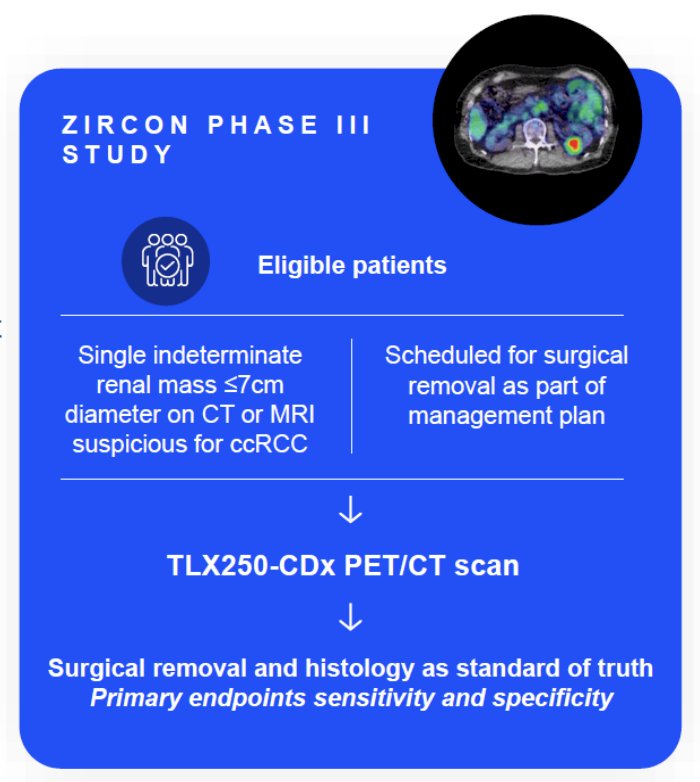
Blinded central histology review determined clear cell RCC status. The co-primary objectives were to evaluate both the sensitivity and specificity of TLX250-CDx PET/CT imaging in detecting clear cell RCC in patients with indeterminate renal masses, using histology as the standard of truth. Key secondary objectives included sensitivity and specificity of TLX250-CDx PET/CT imaging in the subgroup of patients with indeterminate renal masses ≤ 4 cm (cT1a). Other secondary objectives included positive and negative predictive values, safety, and tolerability. There were 300 patients enrolled between August 2019 and August 2022 among 36 sites in 9 countries that received TLX250-CDx. Of 284 evaluable patients included in primary analysis, the average across all 3 readers for sensitivity and specificity was 86% [95% CI 80%, 90%] and 87% [95% CI 79%, 92%], respectively, for co-primary endpoints, and 85% [95% CI 77%, 91%] and 90% [95% CI 79%, 95%], respectively, for key secondary endpoints. For all readers, the lower boundaries of 95% CI for co-primary and key secondary endpoints were > 75%. For all evaluable patients, positive and negative predictive values were ≥ 91.7% and ≥ 73.7%, respectively.
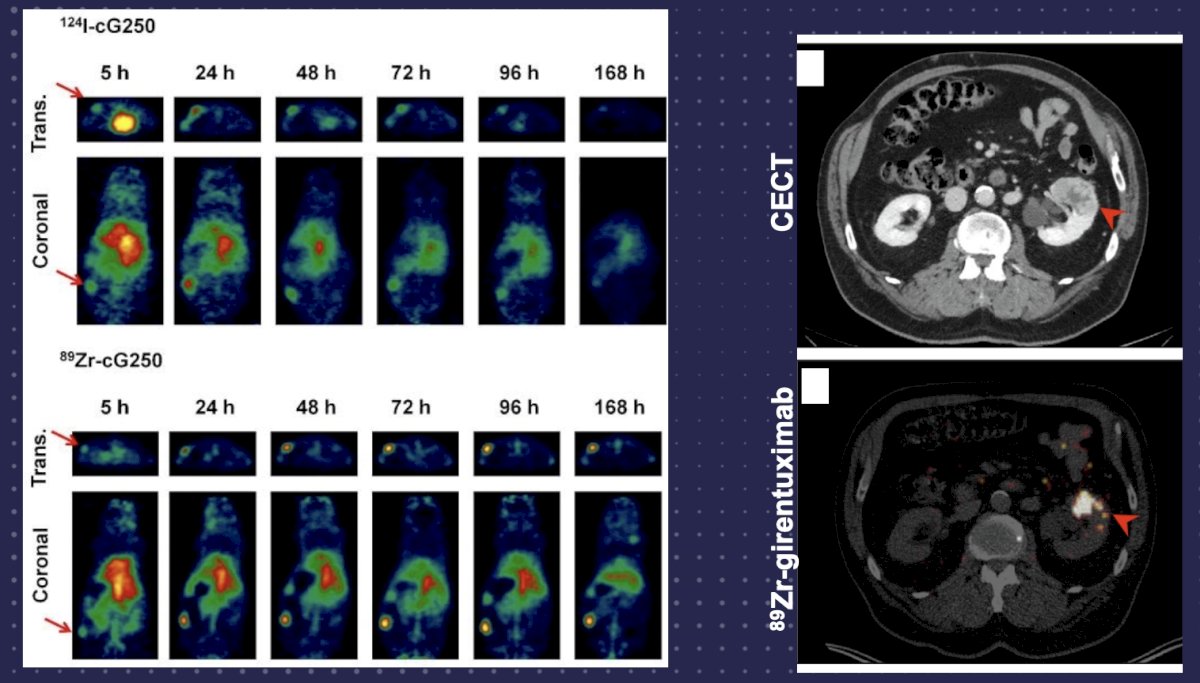
Dr. Bjurlin notes there are several limitations of girentuximab PET:
- It is an antibody based radiotracer with a long-circulating time (interval is 5-7 days, which introduces logistical hurdles to test administration)
- The high cost of PET in general
- Limited availability of PET in certain parts of the world
- Availability is limited by distribution network
- Once approved, this agent will be on a patent for >10 years
- It only tells you clear cell RCC versus all other histologies (benign vs malignant)
99mTc-sestabmibi is a mitochondrial imaging agent used primarily for detecting oncocytomas. Work from Gorin et al.2 assessed 50 patients with a solid clinical T1 renal mass that was imaged with (99m)Tc-sestamibi SPECT/CT prior to surgical resection. Following surgery, 6 (12%) tumors were classified as renal oncocytomas and 2 (4%) as hybrid oncocytic/chromophobe tumors. With the exception of 1 (2%) angiomyolipoma, all other tumors were renal cell carcinomas (82%). (99m)Tc-sestamibi SPECT/CT correctly identified 5 of 6 (83.3%) oncocytomas and 2 of 2 (100%) hybrid oncocytic/chromophobe tumors, resulting in an overall sensitivity of 87.5% (95% CI, 47.4-99.7%). Only two tumors were falsely positive on SPECT/CT, resulting in a specificity of 95.2% (95% CI, 83.8-99.4%):
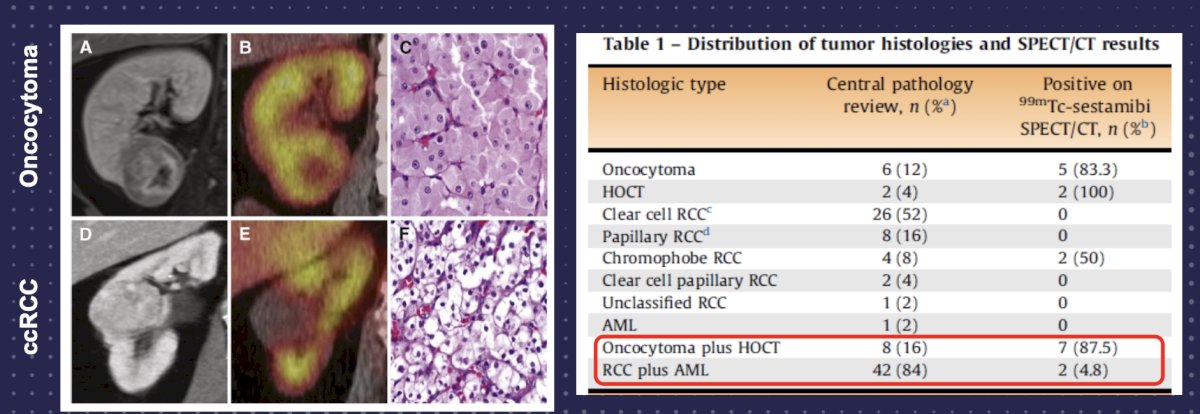
Pooled data among 278 patients and 284 tumors reports a sensitivity of 78.1%, specificity of 91.4%, PPV of 72.5%, and NPV of 93.5% for detection of oncocytoma. Future directions of dual tracer SPECT imaging may be as follows:
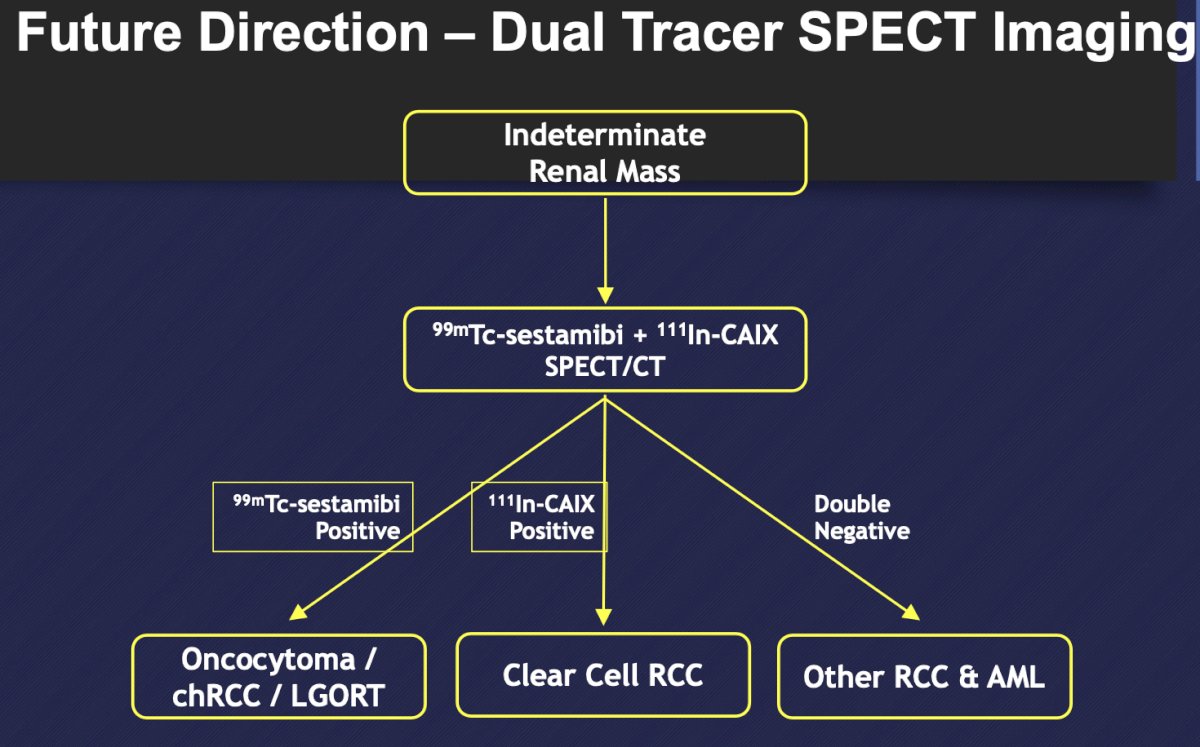
Dr. Bjurlin concluded this section of his talk by discussing molecular imaging for small renal masses with the following thoughts:
- Molecular imaging offers a promising non-invasive means of determining the histology of renal tumors
- 99mTc-sestabmibi SPECT/CT (positive = oncocytoma/oncocytic neoplasm) is an inexpensive and readily available test to aid with the risk stratification of renal tumors, but the literature on this test is limited by single-center studies
- 89Zr-girentuximab/TLX250 PET/CT (positive = clear cell RCC/clear cell papillary RCC) has shown excellent diagnostic performance characteristics in a large multicenter trial, and we should anticipate this test becoming clinically available in the very near future
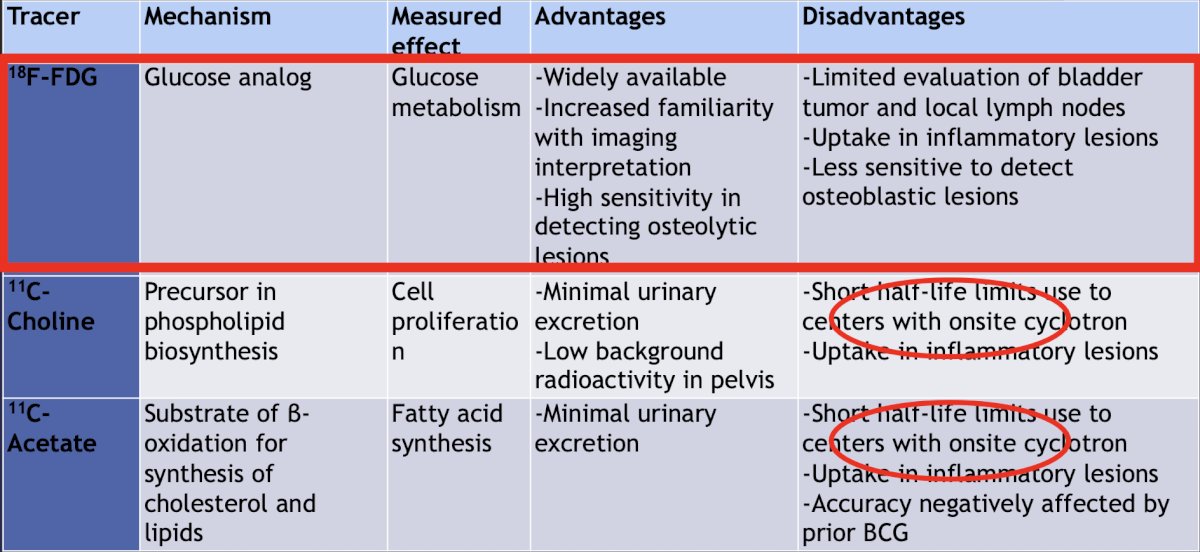
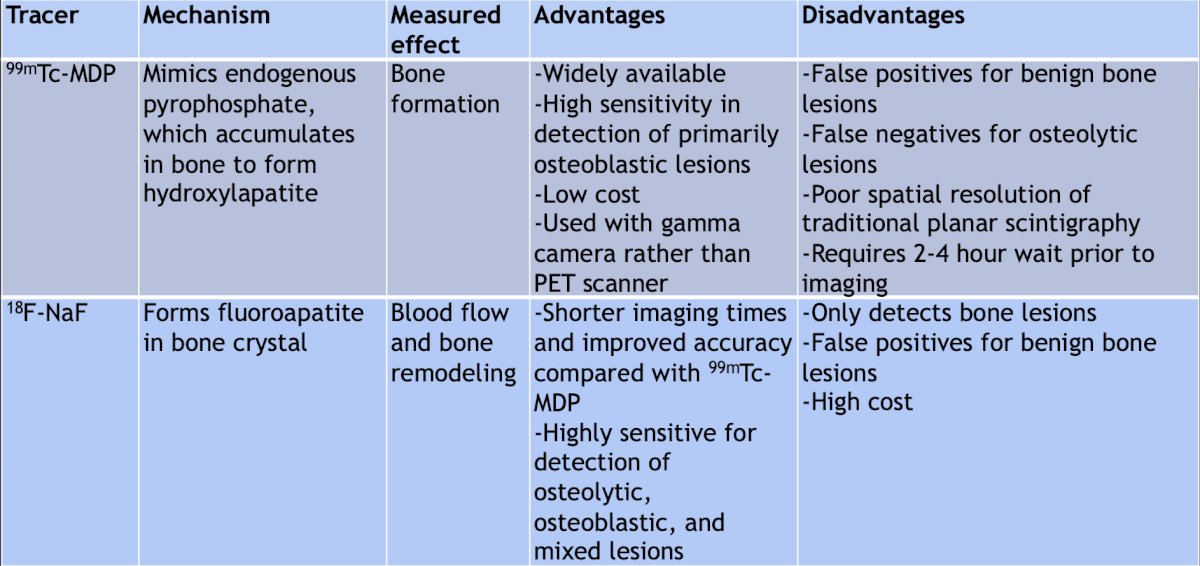
Next, Dr. Bjurlin discussed molecular imaging in bladder cancer, highlighting the advantages and disadvantages of 18F-FDG, 11C-choline, 11C-acetate, 99mTc-MDP, and 18F-NaF:
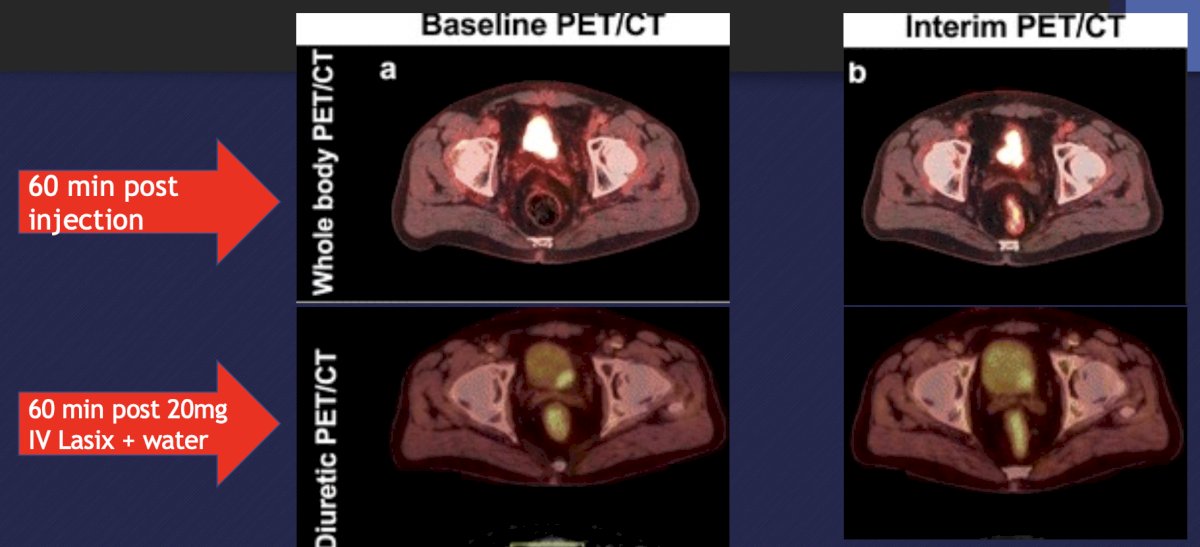
FDG PET provides a limited assessment of the bladder, which can be improved by capturing images 60 minutes after injecting 20 mg of IV Lasix + water:
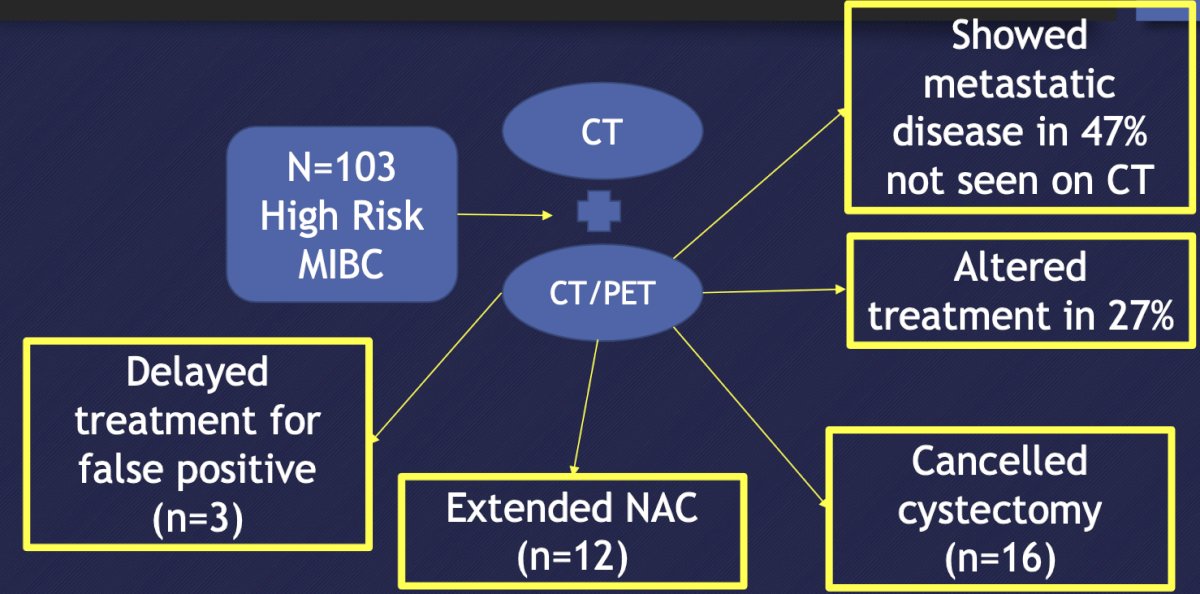
Work from Kollberg et al.3 in 2015 noted that FDG PET/CT improves staging in patients with high-risk muscle-invasive bladder cancer scheduled for radical cystectomy. Among 103 patients, compared to CT alone, FDG-PET/CT provided more additional findings suggesting malignant manifestations in 48 (47%) patients. These additional FDG-PET/CT findings led to an altered provisional treatment plan in 28 (27%) patients, detection of disseminated bladder cancer and subsequent cancellation of the initially intended cystectomy in 16 patients (16%), and identification of disseminated disease and treatment with induction chemotherapy before radical cystectomy in 12 patients (12%):
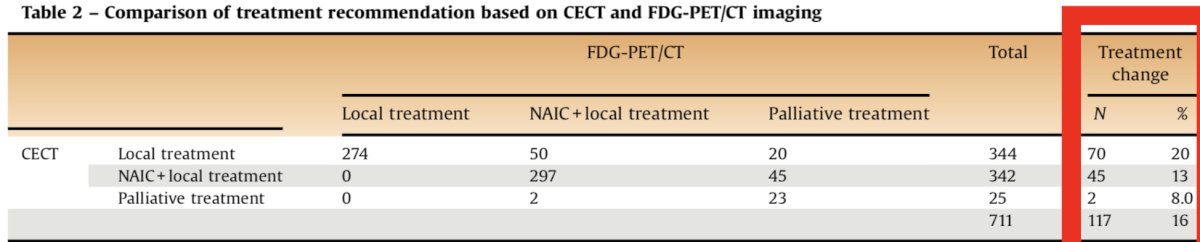
In a retrospective comparison of 711 patients (CT vs FDG PET/CT), clinical stage changed in 26% of patients.4 Upstaging was more frequent than downstaging (25.5% vs 0.4%) and occurred most frequently in patients with cM1a disease (44% upstaging to cM1b):
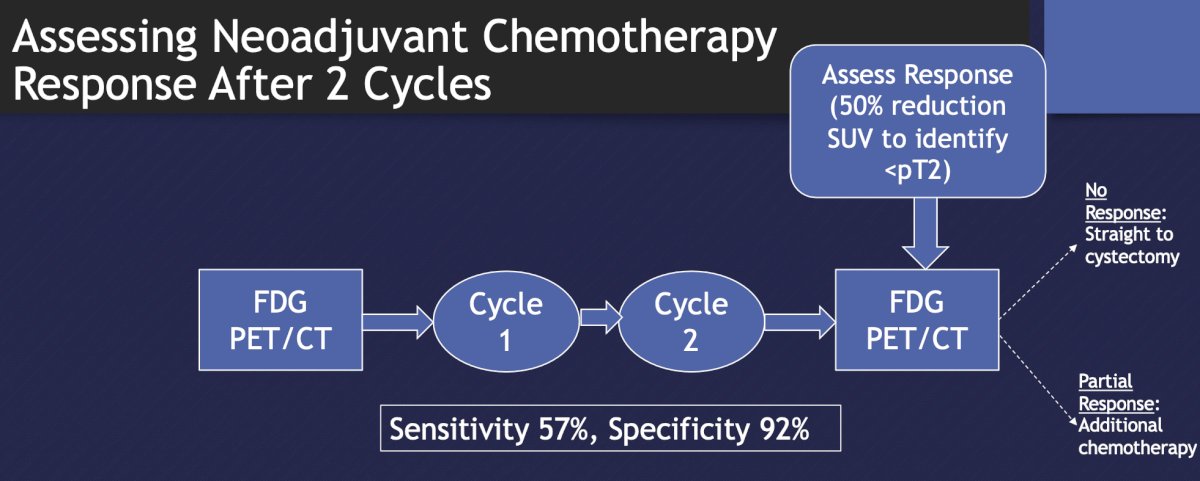
FDG PET/CT can also be used to assess response to 2 cycles of neoadjuvant chemotherapy: for those with no response, they may proceed straight to cystectomy, and for those with partial response, additional chemotherapy can be given (sensitivity 57%, specificity of 92%):
An emerging question regarding radiotracers and immunotherapy is how to predict which patients will respond to anti-PD-(L)1 therapy (given that our current approaches are imperfect). This results in a significant percentage of patients that do not derive clinical benefit, but who are exposed to immune-related adverse events. Thus, it is important to identify responders as early as possible, and avoid unnecessary treatment or toxicity, and allow earlier treatment with other effective agents. Dr. Bjurlin concluded this portion of this talk by discussing molecular imaging in bladder cancer with the following thoughts regarding the pragmatic use of PET/CT in clinical practice:
- For indeterminate lymph nodes or bone lesions
- Patients who cannot get contrasted axial imaging
- Suspected cT3 disease
- High risk histology: plasmacytoid, micropapillary, small cell/neuroendocrine features
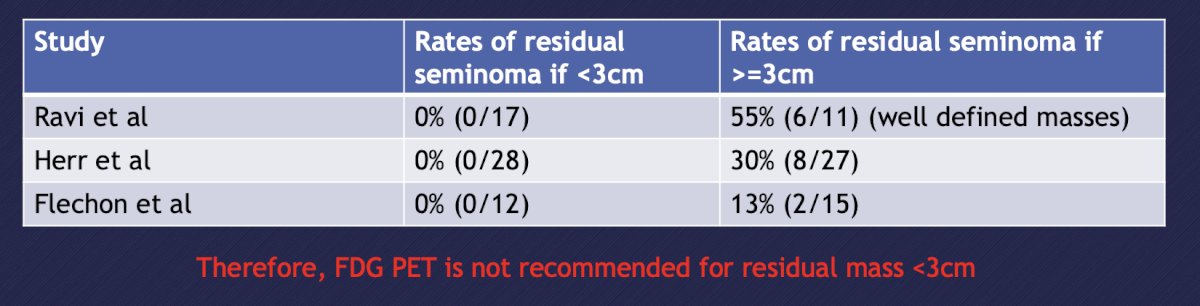
Finally, Dr. Bjurlin discussed the use of PET in the diagnosis and management of germ cell tumors, with slides courtesy of Dr. Rose. The use of PET/CT for initial staging is recommended against in the ESMO and NCCN guidelines. This negative recommendation is secondary to 72 patients (did not meet accrual goal of 169 patients) with clinical stage I/II NSGT undergoing FDG PET prior to primary RPLND. Overall, 19 patients had pathologic nodal involvement (26%) and the primary endpoint was not reached with a negative predictive value of 78%.5 In patients with seminoma, the size of residual masses is used to predict likelihood of viable seminoma after chemotherapy (rate of residual disease in post-chemotherapy seminoma <3 cm is <10%). Based on 3 studies, the rates of residual seminoma if the mass is >= 3 cm ranges from 13-55%:
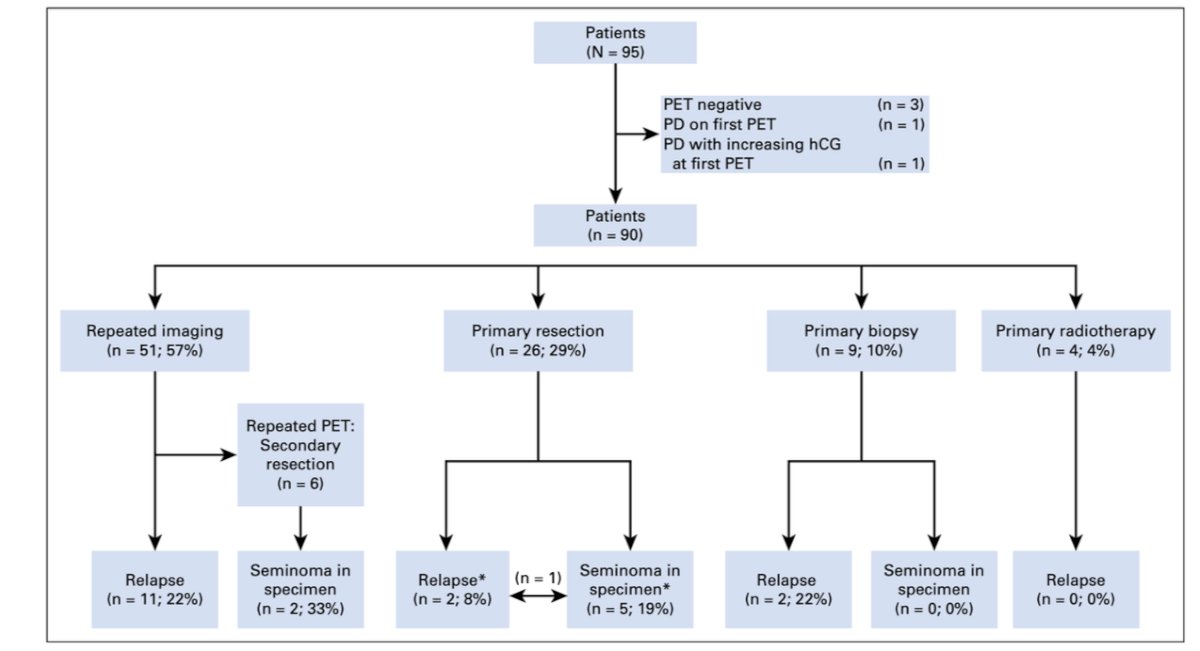
However, the management of FDG PET + post-chemotherapy seminoma >3 cm remains controversial. In 2018, Cathomas et al.6 assessed the International Global Germ Cell Cancer Group Registry (n = 90 with metastatic seminoma with PET-positive residual lesions after chemotherapy), finding that over a median follow-up of 29 months, the median diameter of the largest residual mass was 4.9 cm (range: 1.1 to 14 cm). Post-PET management included repeated imaging in 51 patients (57%), resection in 26 patients (29%), biopsy in nine patients (10%), and radiotherapy in four patients (4%). Histology of the resected specimen was necrosis in 21 patients (81%) and vital seminoma in five patients (19%). Relapse or progression occurred in 15 patients (17%) after a median of 3.7 months (IQR, 2.5 to 4.9 months), and all but one patient who experienced relapse were successfully treated with salvage therapy. The PPV for FDG-PET was only 23%:
Importantly, miR371 (or others) are likely to supplant PET/CT in the evaluation of residual seminoma. Dr. Bjurlin concluded this portion of his talk on molecular imaging in testicular cancer with the following conclusions:
- There is currently no role for PET in NSGCT
- FDG avidity has a high negative predictive value for post-chemotherapy residual seminoma
- Management of FDG avid residual seminoma > 3 cm is nuanced and RPLND and surveillance are options
- PET may be used to predict early treatment response and de-escalated chemotherapy, but this remains investigational
Presented by: Marc Bjurlin, DO, MSc, FACOS, University of North Carolina, Chapel Hill, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Southeastern Section of the American Urological Association (SESAUA) Annual Meeting, Austin, TX, Wed, Mar 20 – Sat, Mar 23, 2024.
References:
- Han S, Woo S, Joon Kim Y, et al. Impact of 68Ga-PSMA PET on the Management of Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. Eur Urol. 2018 Aug;74(2):179-190.
- Gorin MA, Rowe SP, Baras AS, et al. Prospective Evaluation of (99m)Tc-sestamibi SPECT/CT for the Diagnosis of Renal Oncocytomas and Hybrid Oncocytic/Chromophobe Tumors. Eur Urol. 2016 Mar;69(3):413-416.
- Kollberg P, Almquist, Blackberg M, et al. [(18)F]Fluorodeoxyglucose – positron emission tomography/computed tomography improves staging in patients with high-risk muscle-invasive bladder cancer scheduled for radical cystectomy. Scand J Urol. 2015;49(4):296-301.
- Voskuilen CS, van Gennep EJ, Einerhand SMH, et al. Staging 18F-flurodeoxyglucose Positron Emission Tomography/Computed Tomography Changes Treatment Recommendation in Invasive Bladder Cancer. Eur Urol Oncol. 2022 Jun;5(3):366-369.
- de Wit M, Brenner W, Hartmann M, et al. [18F]-FDG-PET in clinical stage I/II non-seminomatous germ cell tumours: of the German multicentre trial. Ann Oncol. 2008 Sep;19(9):1619-1623.
- Cathomas R, Klingbiel D, Bernard B, et al. Questioning the Value of Flurodeoxyglucose Positron Emission Tomography for Residual Lesions After Chemotherapy for Metastatic Seminoma: Results of an International Global Germ Cell Cancer Group Registry. J Clin Oncol. 2018 Oct 4:JCO1800210.


