(UroToday.com) The 2024 Southeastern Section of the AUA (SESAUA) annual meeting featured a prostate cancer session and a presentation by Dr. Daniel George discussing the association of PSA response and overall survival in patients with metastatic hormone-sensitive prostate cancer (mHSPC) in the phase 3 ARASENS trial.1 Reductions in PSA level have been associated with improved overall survival in patients with mHSPC.2,3 At the 2024 SESAUA annual meeting, Dr. George and colleagues presented results for the association between PSA response and overall survival from the ARASENS trial.
Patients were randomized 1:1 to receive darolutamide 600 mg twice daily or placebo, both with ADT + docetaxel. Serum PSA was measured at screening and every 12 weeks:
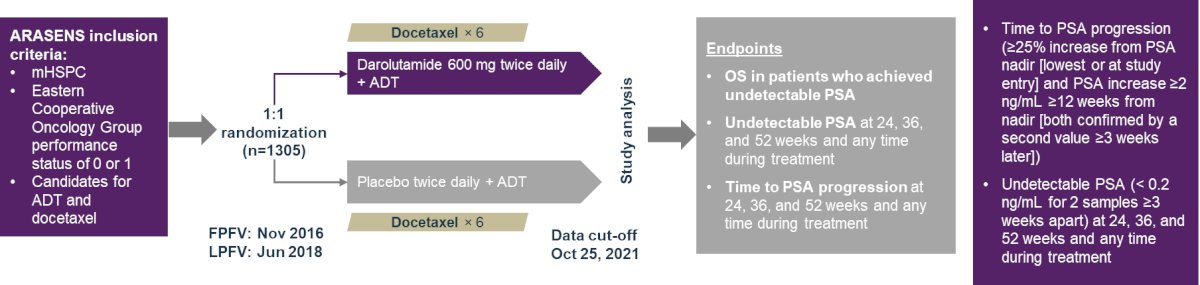
Exploratory analyses included time to PSA progression (≥25% increase from PSA nadir and PSA increase ≥2 ng/mL ≥12 weeks from nadir) and undetectable PSA (<0.2 ng/mL for 2 samples ≥3 weeks apart) at 24, 36, and 52 weeks, and any time during treatment. Between-treatment comparisons were performed using the Cochran–Mantel–Haenszel test. Post hoc analyses evaluated the association between undetectable PSA at weeks 24 and 36, and overall survival for the overall population.
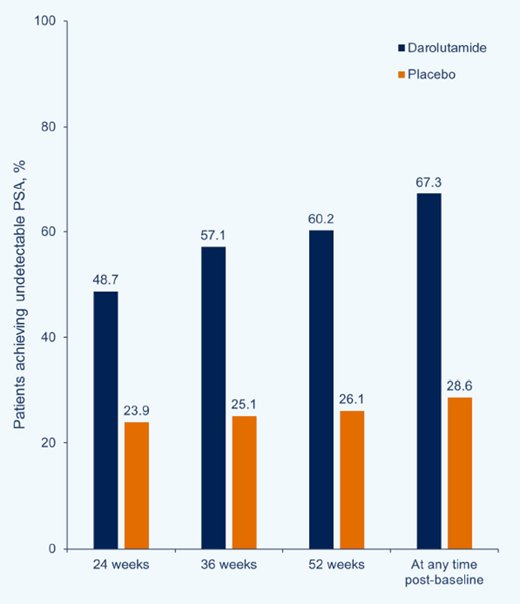
Among 1,306 patients randomized, 1,305 were included in the full analysis (darolutamide n = 651; placebo n = 654). Median PSA levels at study entry were 30.3 ng/mL (range: 0.0–9219.0) and 24.2 ng/mL (range: 0.0–11,947.0), respectively. Undetectable PSA was achieved in more patients receiving darolutamide (48.7%) versus placebo (23.9%) at 24 weeks, increasing to 57.1% and 60.2% at 36 and 52 weeks, respectively, versus minimal change in the placebo group: 25.1% and 26.1%, respectively. Undetectable PSA levels at any time were achieved by 67.3% of patients in the darolutamide group and 28.6% of patients in the placebo group (p < 0.0001) at all time points:
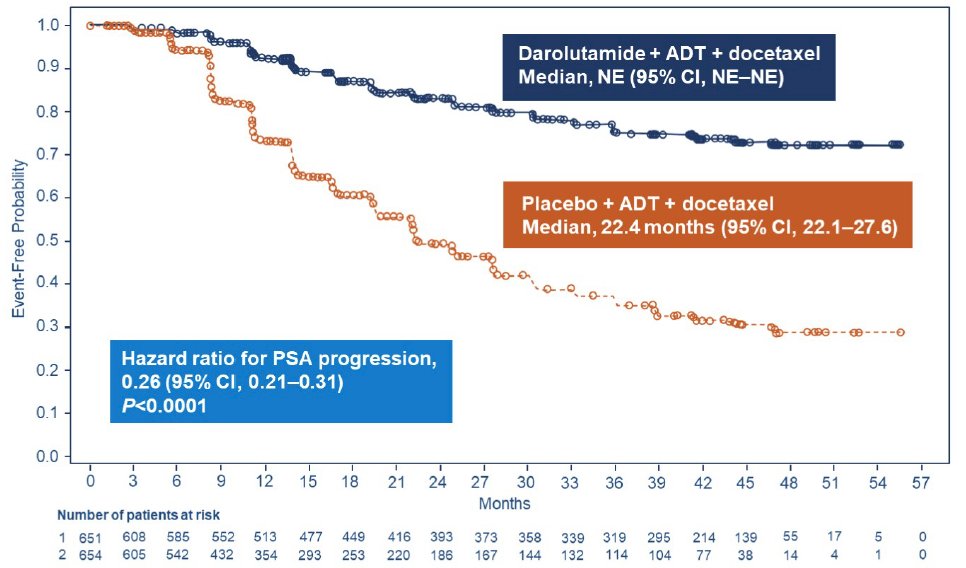
Darolutamide also significantly prolonged the time to PSA progression versus placebo (HR 0.26, 95% CI 0.21-0.31; p < 0.0001):
For the overall population, overall survival was significantly improved for patients who achieved undetectable PSA levels at 24 weeks (HR 0.47, 95% CI: 0.35–0.63) and 36 weeks (HR 0.37, 95% CI: 0.28–0.49) versus those who did not:

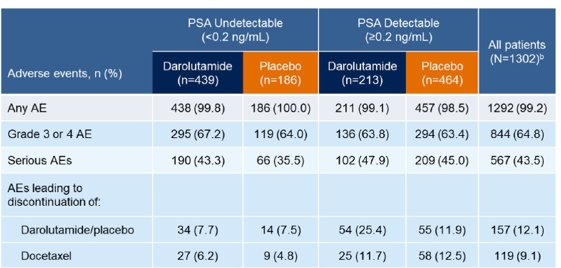
Incidence of adverse events by achievement of undetectable PSA at any time post baseline was as follows:
Dr. George concluded his presentation by discussing the association between PSA response and overall survival in patients with mHSPC in the phase 3 ARASENS trial with the following conclusions:
- More patients receiving darolutamide achieved undetectable PSA levels and had a significant delay in time to PSA progression than those receiving placebo
- In patients with mHSPC treated with darolutamide in combination with ADT and docetaxel, achievement of undetectable PSA was associated with improved overall survival
- Risk of death reduced by 53% and 63%, respectively, versus those who did not achieve undetectable PSA level at 24 and 36 weeks
Presented by: Daniel J. George, MD, Duke Cancer Institute, Durham, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Southeastern Section of the American Urological Association (SESAUA) Annual Meeting, Austin, TX, Wed, Mar 20 – Sat, Mar 23, 2024.
References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Harshman LC, Chen YH, Liu G, et al. Seven-Month Prostate-Specific Antigen is Prognostic in Metastatic Hormone-Sensitive Prostate Cancer Treated with Androgen Deprivation with or without Docetaxel. J Clin Oncol. 2018 Feb 1;36(4):376-382.
- Matsubara N, Chi KN, Ozguroglu M, et al. Correlation of Prostate-specific Antigen Kinetics with Overall Survival and Radiological Progression-free Survival in Metastatic Castration-sensitive Prostate Cancer Treated with Abiraterone Acetate plus Prednisone or Placebos Added to Androgen Deprivation Therapy: Post Hoc Analysis of Phase 3 LATITUDE Study. Eur Urol. 2020 Apr;77(4):494-500.


