However, aside from the testicle, FSH has targets in other regions and cells of the body. These include the adipocytes (fat cells), hepatocytes (liver cells), osteoclasts (bone resorption cells), and macrophages in the blood and connective tissue. These extragonadal targets represent the side effect profile of androgen deprivation therapy (ADT) given for advanced prostate cancer disease. FSH has also potential mitogenic signaling. It has been also shown that FSH levels predict time to development of castration-resistant prostate cancer (CRPC) among men treated with ADT. Higher levels of FSH result in a shorter time to development of CRPC and worse prognosis.1 Another study has shown that higher FSH levels are also associated with the extraprostatic extension of prostate cancer.2
All forms of ADT (orchiectomy, GnRH agonists and antagonists) cause castration with a significant reduction of testosterone levels. However, ADT in the form of orchiectomy causes a significant increase in FSH levels, as there is no more negative feedback on GnRH secretion. In contrast, GnRH agonists and antagonists cause a decrease in FSH levels (more substantially in GnRH antagonists).
As mentioned before, FSH induces fat accumulation in the adipocytes. This has been shown both in in-vitro models and in-vivo. FSH also causes a reduction in the clearance of low-density lipoproteins (LDL) in the hepatocytes, by inhibiting the expression of the receptor of LDL and thus reducing cholesterol clearance. Moreover, FSH induces Non-alcoholic fatty liver disease (NAFLD, or hepatosteatosis). Another important effect of high FSH levels, is the stimulation of osteoclastogenesis and bone resorption. The FSH receptor is expressed on adipocytes, prostate, skeletal muscle, cardiac myocytes, testis, and ovary. That is why high FSH levels have also been associated with more cardiovascular morbidity. Lastly, FSH has also been shown to cause foam cell development from macrophages. All these lead to the fact that high FSH level, in the context of testosterone elimination, accelerate atherosclerosis, resulting in worse cardiovascular disease. When examining the differential effects of common primary ADT modalities on atherosclerosis in-vivo, it is clear that GnRH agonists cause the largest atherosclerotic plaques, while GnRH antagonists cause the smallest ones (Figure 1).
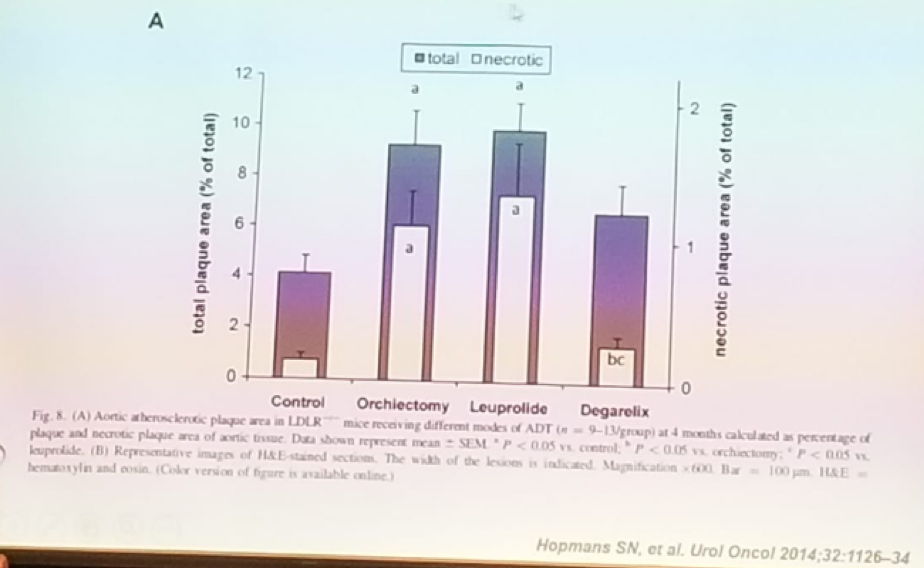
Figure 1: Differential effects of common primary ADT modalities on atherosclerosis in-vivo:
Dr. Pinthus summarized this talk, stating that FSH has potential oncogenic effects in prostate cancer. Different ADT modalities have different effects on FSH levels. Lastly, FSH effects multiple metabolic pathways, which link to the adverse effects of ADT.
Presented by: Jehonathan H. Pinthus, MD, Ph.D., FRCSC, McMaster University, Hamilton, Ontario, Canada
References:
1. Hoare D et al. CUAJ 2015
2. Hisamitsu I et al. Prostate Int. 2013
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre Twitter: @GoldbergHanan at the 38th Congress of the Society of International Urology - October 4- 7, 2018 - Seoul, South Korea


