In 2012 the US Preventive Services Task Force (USPSTF) gave a “D” recommendation against prostate cancer screening, basically stating that it should not be offered to patients. In May 2018 they altered it to a “C” recommendation. Beyond the changing USPSTF recommendations, there is a large variety of biomarkers and tests available on the market today for prostate cancer diagnosis, resulting in great confusion among the primary physicians, who do most of the initial prostate cancer screening today. PSA cutoffs have changed from 4 to 2.5 ng/ml, additional PSA tools have been introduced, including PSA velocity and density, and various new biomarkers have been available on the market, including 4K test, SelectMDx, PCA3, phi, and others. All these add to the confusion faced by the primary physicians, who are simply in search of a simple pathway of how to manage these patients.
According to Dr. Crawford, we need to simplify the message for the primary physicians. In an update and review of the PLCO trial, one of the largest prostate cancer screening studies (flawed by contamination in the non-screening arm), it was shown that patients who had an initial PSA < 1.0 ng/mL had a very low rate of prostate cancer developing in the following 5 years.1 This was the first substantial evidence proving that if we lower the PSA cut-off, it could help improve PSA screening. In an attempt to explore this further, Dr. Crawford analyzed 350,000 patients in the Henry Ford HMO system to determine what initial PSA values could tell us about future prostate cancer risk.2 The results demonstrated that the median PSA was 1.0 ng/ml, and the mean age of patients examined was 55, with 30% of them being African-American. Importantly, he demonstrated that patients with PSA < 1.5 ng/ml had a 0.51% risk of developing prostate cancer. When PSA was between 1.5-4.0, the risk increased 15-fold to 7.85%, and in African-Americans, it increased even further to 10.39% (Figure 1). Dr. Crawford concluded that the PSA range of 1.5-4.0 was a “danger zone” and mandated closer follow-up.
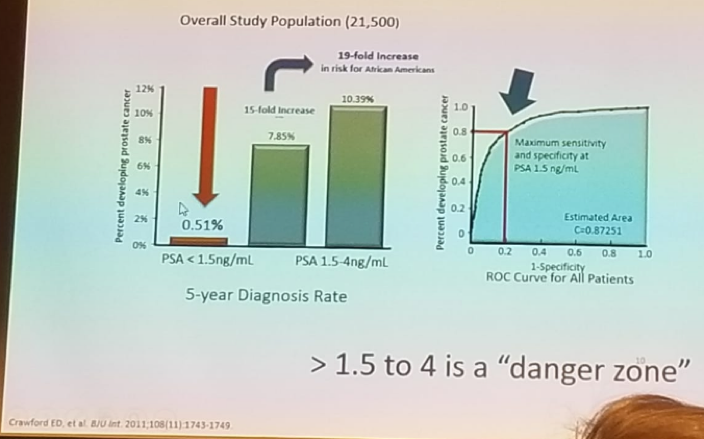
Figure 1 – PSA 1.5-4 ng/ml is the “Danger Zone”:
A PSA of more than 1.5 ng/ml is a surrogate for benign prostatic hyperplasia (BPH) (most commonly), prostate cancer, and long-term prostate cancer risk. Dr. Crawford emphasized that this does not mean that everyone with PSA > 1.5 ng/ml should get a biopsy. It just means that these patients need to be evaluated further for prostate cancer risk, with additional tests and biomarkers. The data demonstrated that only 20% of patients had a PSA> 1.5 ng/ml, so not all men will be subjected to unneeded evaluation.
Next, Dr. Crawford discussed how we can be certain that we are not missing significant prostate cancer in men with PSA < 1.5 ng/ml. He showed data from a recently published study by the University of Toronto, demonstrating the results of an aggressive prostate biopsy in younger men (age < 50).3 In these men, a PSA of 1.5 ng/ml remained an important cutoff. Clinically significant cancer was diagnosed only when PSA was above 1.5 ng/ml (Figure 2).

Figure 2 – Study demonstrating the results of an aggressive prostate biopsy in men younger than 50 stratified by PSA values, demonstrating clinically significant disease only when PSA> 1.5 ng/ml:
Goldberg et al. J. Urol 2018
Lastly, Dr. Crawford reviewed the correlation between common biomarkers (4K, PHI, SelectMDx) and a PSA of less than 1.5 ng/ml. This showed that in men with a PSA < 1.5 ng/ml, the biomarkers were rarely positive, validating the sense that these men do not need to be further evaluated.
Dr. Crawford emphasized that the time when PSA alone guided prostate biopsy decisions has ended. We need better risk assessments to detect clinically significant cancers and reduce unnecessary biopsies and over-detection of indolent disease. He believes that this can be achieved with genomic biomarkers. Therefore, A final algorithm created by Dr. Crawford was proposed. According to this algorithm, when the PSA < 1.5 ng/ml, the patient should not get a repeat PSA for at least 5 years. When the PSA >1.5 ng/ml, the primary physicians should consider a referral to a urologist. The urologist at this time should consider using genomic biomarkers, and if they demonstrate high-risk results, a biopsy should be performed.
In summary, PSA of 1.5 ng/ml is a reasonable cut-off, although it is not perfect. It harbors a simple message to the primary physicians to remember. A PSA of 1.5-4 ng/ml is a grey zone, harboring many potential diagnoses, including BPH, prostatitis, or cancer. Lastly, clinical evaluation and genomic markers will help determine whom to biopsy.
Presented by: E. David Crawford, MD, Professor, Radiation Oncology, Univesity of Colorado, USA
References:
1. Andriole et al, NEJM 2009
2. Crawford ED, et al. BJU Int. 2011
3. Goldberg H, et al. J Urol 2018
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre Twitter: @GoldbergHanan at the 38th Congress of the Society of International Urology - October 4- 7, 2018 - Seoul, South Korea


