Athens, Greece (UroToday.com) Dr. Renu Eapen gave an overview of the role of PET- prostate-specific membrane antigen (PSMA) in prostate cancer and gave a preview of what is to come in the near future of PET diagnostics, and the evolving field of theranostics.
PSMA is a transmembrane glycoprotein with folate hydrolase activity. It is highly specific for benign and malignant prostate cells. It is overexpressed in prostate cancer cells, and especially in metastatic and castrate-resistant disease (CRPC).
Lutetium-177 is a short path-length beta emitter. It has a crossfire effect and targets all cells in a 1 mm radius. It allows effective delivery of radiation to tumors while minimizing damage to surrounding normal tissues. The Lu-PSMA is a variant of 68-Ga-PSMA-11 used for PET imaging, and it is optimized for therapeutic use (Figure 1). It was initially developed by the German Cancer Research Center and University Hospital Heidelberg. Its attributes include rapid plasma clearance and higher affinity binding to PSMA.
Figure 1 – The mechanism of therapy with lutetium-177-PSMA1
The first reported use of lutetium-PSMA for treating prostate cancer was in 20152 and since then, its use has been growing significantly all over the world, including Australia (Figure 2). A landmark Australian study on the use of Lu-PSMA was published in Lancet Oncology in 2018.3 This was the first prospective phase II trial taking place between 2015 and 2018. Remarkable responses were seen in patients who progressed after conventional therapies. The inclusion, exclusion and primary endpoints of this study are seen in figure 3. A total of 50 patients were finally analyzed, and the results were quite impressive showing a PSA response rate of more than 80% in 44% of patients (Table 1)
Figure 2 – Lu-PSMA increased us in the Peter MacCallum Center in Australia
Figure 3 – Inclusion, exclusion criteria and primary endpoints of the Lu-PSMA study

Table 1 – Initial results of the Lu-PSMA study
When assessing the toxicity, the treatment was relatively very well-tolerated with mostly grade 1-2 adverse effects. The most common were dry mouth (58%), nausea (40%), fatigue (30%), and thrombocytopenia (22%).
It is now being considered to use Lu-PSMA treatment at an even earlier stage of prostate cancer (Figure 4), and as a result, there are several randomized prospective trials in the pipeline coming from Australia, attempting to assess the role of Lu-PSMA in various prostate cancer scenarios.
Figure 4 – When can we use Lu-PSMA?
The PRINCE and LuPARP trials will assess the role of Lu-PSMA in metastatic CRPC patients (Figures 5, 6, and 7). The UpFrontPSMA trial will assess the role of this treatment in metastatic hormone-sensitive prostate cancer in comparison to androgen deprivation therapy + docetaxel (Figure 8), and the LUTECTOMY trial will assess its role in the neoadjuvant setting, prior to radical prostatectomy, in patients with high-risk localized prostate cancer (Figure 9).
Figure 5 – PRINCE trial – assessing Lu-PSMA in the mCRPC setting
Figure 6 – LuPARP trial - assessing Lu-PSMA in the mCRPC setting
Figure 7 - TheraP trial - assessing Lu-PSMA in the mCRPC setting
Figure 8 – UpFrontPSMA trial - assessing Lu-PSMA in the metastatic hormone-sensitive setting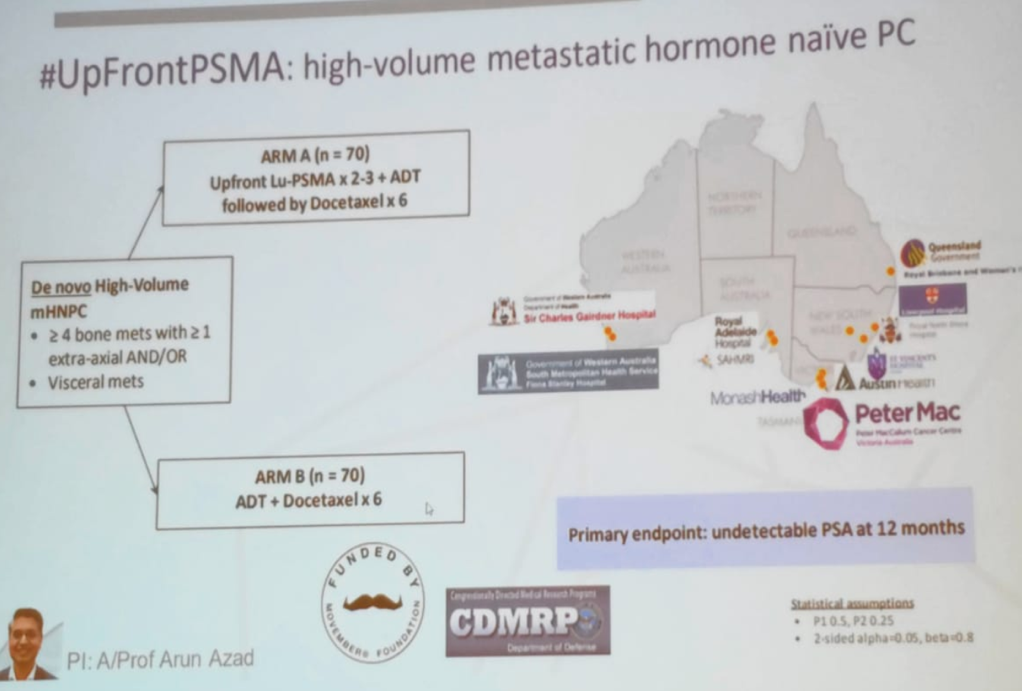
Figure 9 – LUTECTOMY trial - assessing Lu-PSMA in the neoadjuvant setting before radical prostatectomy for high-risk localized prostate cancer
Summarizing her talk, Dr. Eapen stated that it is important to note that Lu-PSMA is not suitable for all patients. Suitable patients are those with PSMA-PET response and FDG+ concordant or no FDG-PET response at all. In contrast, patients with no PSMA response and those who are PSMA positive but FDG+ discordant are not suitable for this treatment (Figure 10). Clearly, there is a lot to look forward to in this extensively growing field of theranostics.
Figure 10 -Who is suitable for Lu-PSMA treatment?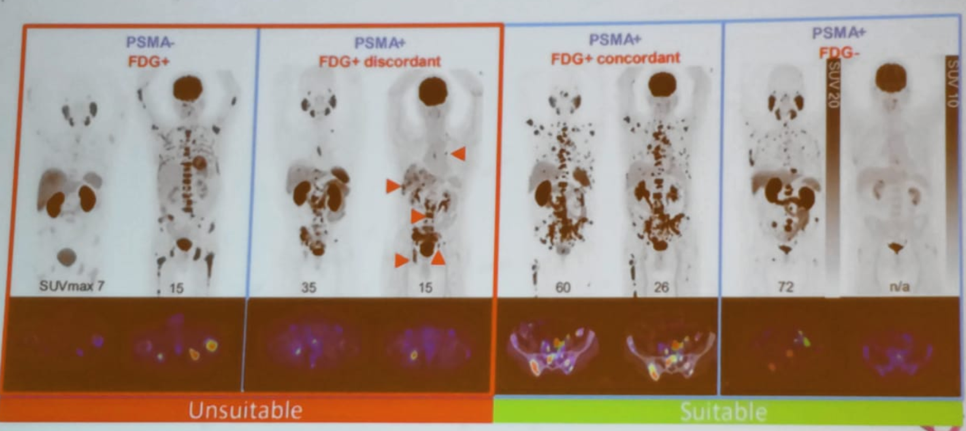
Presented by: Renu Eapen, FRACS, Consultant Urologist, Genitourinary Oncology Service, Peter MacCallum Cancer Center, Australia
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New-York, USA @GoldbergHanan at the 39th Congress of the Société Internationale d'Urologie, SIU 2019, #SIUWorld #SIU2019, October 17-20, 2019, Athens, Greece
References:
- Ferdinandus J, Violet J, Sandhu S, Hofman MS. Prostate-specific membrane antigen theranostics: therapy with lutetium-177. Current opinion in urology 2018; 28(2): 197-204.
- Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2016; 57(8): 1170-6.
- Hofman MS, Violet J, Hicks RJ, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. The Lancet Oncology 2018; 19(6): 825-33.


