Athens, Greece (UroToday.com) Dr. Ricardo Rendon presented on the adjuvant treatment of high-risk renal cell carcinoma (RCC). The cancer-specific survival (CSS) for localized RCC following surgical resection is 84% at 5 years, and 76% at 10 years. However, for patients with high-risk features, the CSS is around 30-40%.
The optimal treatment strategy for high-risk RCC patients include:
- Surgery
- Effective anticancer perioperative systemic treatment (eradicate micrometastatic disease)
- Having minimal toxicity
Multiple factors play a role in determining if adjuvant therapy is appropriate in individual cases. These include:
- Risk of disease relapse
- Rate of response to the agent
- Risk of toxicity to therapy
There are multiple nomograms incorporating attempting to help us ascertain the patient’s risk factors:
- American Joint committee on cancer (AJCC) TNM staging
- Eastern Cooperative Oncology Group (ECOG) performance status
- Tumor size
- Histologic features:
- Fuhrman nuclear grade
- Presence or absence of necrosis
The most commonly used nomograms are the Leibovich score (Figure 1), and the UCLA integrated staging system (UISS) score (Figure 2).
Figure 1 – Leibovich Score (specifically designed to predict disease-free survival after surgery for patients with localized clear-cell RCC):

Figure 2 – UCLA Integrated Staging System (UISS (for clear cell and non-clear cell RCC):

Next, Dr. Rendon discusses on the adjuvant therapy in RCC. Dr. Rendon showed Figure 3, which consisted of several randomized trials that have assessed Anti VEGF and mTOR inhibitors in the adjuvant setting.
Figure 3 – Randomized trials of Anti-VEGF and mTOR adjuvant therapy in renal cell carcinoma:

One of these studies is the S-TRAC study,1 which randomized patients to placebo and sunitinib in clear-cell RCC patients 3-12 weeks after radical nephrectomy. All patients had loco-regional RCC with an ECOG<=2 before nephrectomy. This study demonstrated a disease-free-survival (DFS) advantage for patients treated with sunitinib compared to placebo (HR 0.76, 95% CI 0.59-0.98). Unfortunately, the data was not mature at the time of cutoff, and it did not meet the pre-specified criteria for the minimally important difference in overall survival. When assessing the safety results, the rate of adverse effects was significantly higher in patients treated with sunitinib. The S-TRAC was the only study to show a statistically significant DFS benefit for sunitinib. This resulted in the FDA approving sunitinib for high-risk patients in 2017 as adjuvant therapy for RCC. In contrast, the European Medicine Agency (EMA) recommended against this drug given the low benefit to risk ratio.
The next trial discussed by Dr. Rendon was the latest adjuvant trial, named the SORCE trial (RE05), which was an international randomized double-blind phase III trial. This trial assessed adjuvant sorafenib for RCC at intermediate or high risk of relapse. This trial was presented recently at ESMO 2019, in Barcelona. This study did not show a difference between sorafenib and placebo with a DFS HR of 1.01 (95% CI 0.82-1.23) after three years.
Almost all these anti-VEGF and mTOR trials have been negative. Possible reasons for this include:
- The dose of medication given or
- Patient selection or
- Mechanism of action
The percent of patients receiving a complete dose in these studies varied between 13-100%. When doing sub-analyses that included only patients who received a higher dose in the PROTECT2 and ASSURE3 trials, the analysis in the PROTECT study showed an improvement in DFS in the 800 mg group, compared to the 600 mg group (31% vs. 14%). In contrast, in the ASSURE trial, no differences were shown.
When assessing patient selection, it was thought that the results were not positive as these studies did not only include high-risk patients. Therefore, sub-analyses were performed to only include high-risk patients. In both the ASSURE3 and the ATLAS4 trials the results did not seem to be different in these sub-analyses.
Lastly, the mechanism of action of these drugs was assessed. Antiangiogenic work through several mechanisms acts against this cancer:
- Blocking neovascularization
- Vascular trimming
- Intratumor hypoxia
- Blocking of tumor expansion
An important difference between overt metastases and micrometastases is the degree of vascularity. While in overt metastases the degree of vascularity is very high, in micrometastases the degree of vascularity is very minute, therefore angiogenic medication would not work, possibly explaining the negative results of these trials.
In the next part of his talk, Dr. Rendon discussed on immune checkpoint inhibitors. The rationale for this medication in RCC patients includes:
- Immune checkpoint inhibitors have shown durable efficacy with a favorable safety and tolerability profile.
- These medications have tumor-directed cytotoxic effects that may eradicate residual micrometastatic disease and prevent disease relapse.
- Experience in melanoma indicates that these medications in the adjuvant setting could be a promising strategy
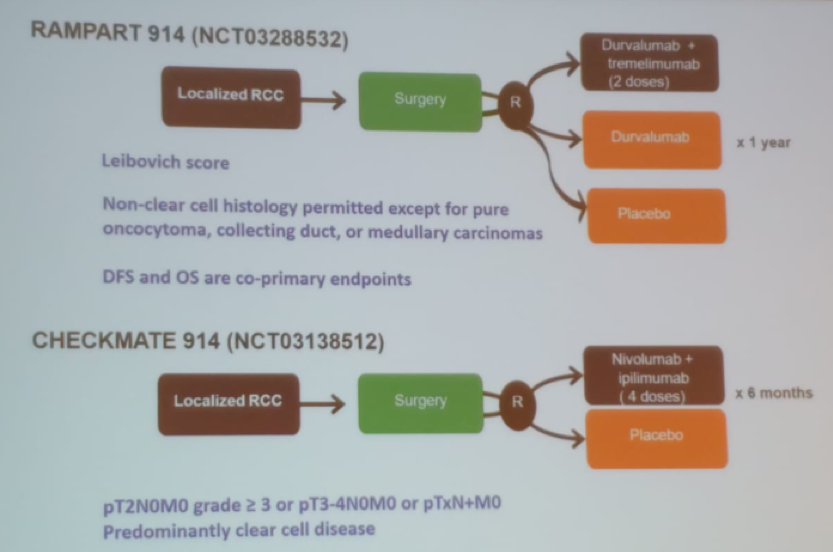
Several randomized trials have been performed to assess the role of these medications in RCC patients (Figure 4).
Figure 4 – Randomized controlled trials assessing the role of immune checkpoint inhibitors in RCC:

The last topic discussed by Dr. Rendon was the role of biomarkers in this disease. Predictive biomarkers are lacking for treatment efficacy and toxicity. Using immune checkpoint inhibitors-associated predictive biomarkers could potentially:
- Be specific to treatment regimens
- Cannot be generalized across disease types and treatments
- Have a possible correlation between immune-mediated adverse effects and treatment efficacy
Concluding his talk, Dr. Rendon stated that risk stratification with clinical factors is inaccurate in RCC patients. Nomograms incorporating gene expression appear to perform better. The era of VEGF in the adjuvant setting is probably at an end. Currently, there is great optimism with the therapeutic option of immune checkpoint inhibitors, whether as monotherapy or as various combinations. Lastly, further development of effective biomarkers is necessary for patient stratification and to improve the efficacy of systemic therapy in the adjuvant setting.
Presented by: Ricardo A. Rendon, MD, Director of the Department of Urology Clinical Trials Unit, Chair of the Department of Urology Research Committee, Dalhousie University, Halifax, Canada
Written by: Hanan Goldberg, MD, Urology Department, SUNY Upstate Medical University, Syracuse, New-York, USA @GoldbergHanan at the 39th Congress of the Société Internationale d'Urologie, SIU 2019, #SIUWorld #SIU2019, October 17-20, 2019, Athens, Greece
References:
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. The New England journal of medicine 2016; 375(23): 2246-54.
- Motzer RJ, Haas NB, Donskov F, et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 2017; 35(35): 3916-23.
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. The Lancet 2016; 387(10032): 2008-16.
- Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Annals of oncology : official journal of the European Society for Medical Oncology 2018; 29(12): 2371-8.


