Dr. Roupret started by noting that urothelial carcinoma is a model for high mutation tumor burden. When comparing bladder urothelial carcinoma to UTUC, Dr. Roupret suggests that they are “disparate twins” as highlighted in the following summary table:
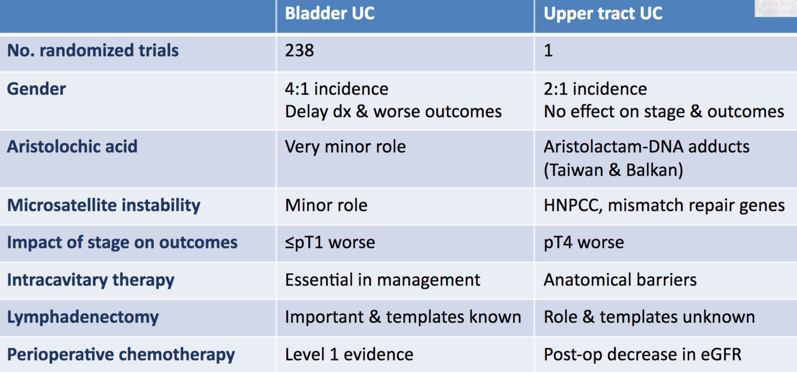
Bladder urothelial carcinoma and UTUC share many features but are distinct diseases, as there are practical, anatomical, biological, and molecular differences that warrant consideration in clinical decision-making. The molecular subtype classification of urothelial carcinoma in Lynch syndrome is associated with MSI positivity but shares similarities with sporadic urothelial carcinoma. In a comprehensive molecular characterization of UTUC, Moss et al.1 used whole-exome sequencing (n=31) to identify mutations in FGFR3 (74.1%; 92% low-grade, 60% high-grade), KMT2D (44.4%), PIK3CA (25.9%), and TP53 (22.2%). They subsequently were able to generate four molecular subtypes with the following characteristics:
- Cluster 1: no PIK3CA mutations, nonsmokers, high-grade <pT2 tumors, high recurrences
- Cluster 2: 100% FGFR3 mutations, low-grade tumors, tobacco use, noninvasive disease, no bladder recurrences
- Cluster 3: 100% FGFR3 mutations, 71% PIK3CA, no TP53 mutations, five bladder recurrences, tobacco use, tumors all <pT2
- Cluster 4: KMT2D (62.5%), FGFR3 (50%), TP53 (50%) mutations, no PIK3CA mutations, high-grade pT2+ disease, tobacco use, carcinoma in situ, shorter survival; identification of a novel SH3KBP1-CNTNAP5 fusion
With regards to neoadjuvant chemotherapy in UTUC, the pros are that the patient has better renal function, and there is evidence for benefit in the patients with bladder urothelial carcinoma. Cons of neoadjuvant chemotherapy are that there is no definitive pathology preoperatively, given that UTUC is difficult to accurately stage, this may lead to overtreatment of some patients. The EA 8141 study assessed prospectively neoadjuvant chemotherapy in patients with UTUC, noting that in Arm A (n=29; adjuvant MVAC), there was a 14% complete response rate, and 62% downstaging rate to ≤pT1. The conclusions from this small phase II trial were that it is feasible to recruit to a multicenter cisplatin neoadjuvant trial for UTUC and that adjuvant MVAC was safe, well tolerated and given the pCR/downstaging rates should be further evaluated in clinical trials.
When looking at immunotherapy in UTUC, Dr. Roupret notes that there are no designated trials for PD-1 inhibitors in UTUC, and we are currently relying on urothelial carcinoma trials that have a subset of UTUC in the trial. For example:
- IMvigor 210: 21% UTUC of 310 M+ patients treated with atezolizumab
- IMvigor 211: 27% UTUC of 467 M+ patients treated with atezolizumab
- KEYNOTE-045: 14% UTUC of 270 M+ patients treated with pembrolizumab
There has been no data on differences in response to PD-1 inhibitors for UTUC vs bladder cancer, and no data on tumor mutational burden in UTUC patients in these aforementioned clinical trials.
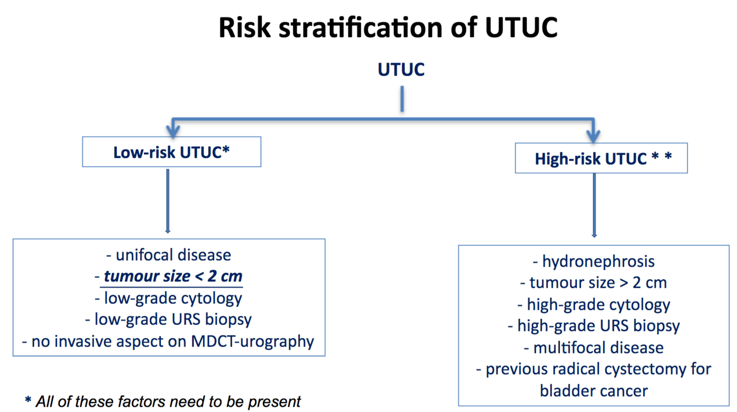
Accurately designating low and high-risk UTUC is crucial for potentially offering patients nephron-sparing surgical treatment, with the following algorithm provided by Dr. Roupret:
Other procedural possibilities for improving detection of high-grade disease include confocal laser endomicroscopy (Cellvizio®), which provides real-time confocal laser endomicroscopy in vivo microscopic images of tissues using a low-energy laser light source.
Finally, Dr. Roupret discussed the use of a single post-operative dose of chemotherapy after radical nephroureterectomy to decrease the chance of bladder recurrence. According to the EAU guidelines,3 there is grade B evidence for post-operative chemotherapy instillation. Dr. Roupret also discussed the recently published OLYMPUS trial,4 which was a phase 3, open-label, single-arm trial designed to assess the efficacy, safety, and tolerability of UGN-101 (a mitomycin-containing reverse thermal gel) in patients with low grade, noninvasive upper tract urothelial cancer:
Among 110 patients screening, 74 were enrolled and 71 patients received treatment. Of the 71 patients who received at least one dose of the study medication, 61 completed the 6 treatments defining the initial treatment. Among those who discontinued treated, this was due to adverse events in 9 patients and personal reasons in the remaining one. There were 42 patients (59%, 95% CI 47-71%) that had a complete response at the time of primary disease evaluation. Of the remainder, 8 (11%) had a partial response, 12 (17%) had no response, 6 (8%) had newly diagnosed high-grade disease, and 3 (4%) had an indeterminate response. Twelve-month durability could be assessed in 20 patients, and of these patients, 14 (70%) showed ongoing durability of their complete response and 6 had a documented recurrence during follow-up. This is encouraging data for UGN-101, that will continue to be assessed in future trials.
Presented by: Morgan Roupret, MD, PhD, Professor of Urology, Sorbonne Université, Paris (UPMC), ESOU chairman, Paris, France
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md
References:
- Moss TJ, Qi Y, Xi L, et al. Comprehensive Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol 2017 Oct;72(4):641-649.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomized controlled trial. Lancet 2020 Mar 5 [Epub ahead of print].
- Roupret M, Babjuk M, Capoun O, et al. European Association of Urology Guidelines on Upper Tract Urothelial Carcinoma: 2020 Update. Eur Urol 2020 Jun 24:S0302-2838(20)30427-9.
- Kleinmann N, Matin SF, Pierorazio PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): An open-label, single-arm, phase 3 trial. Lancet Oncol 2020 Jun;21(6):776-785.


