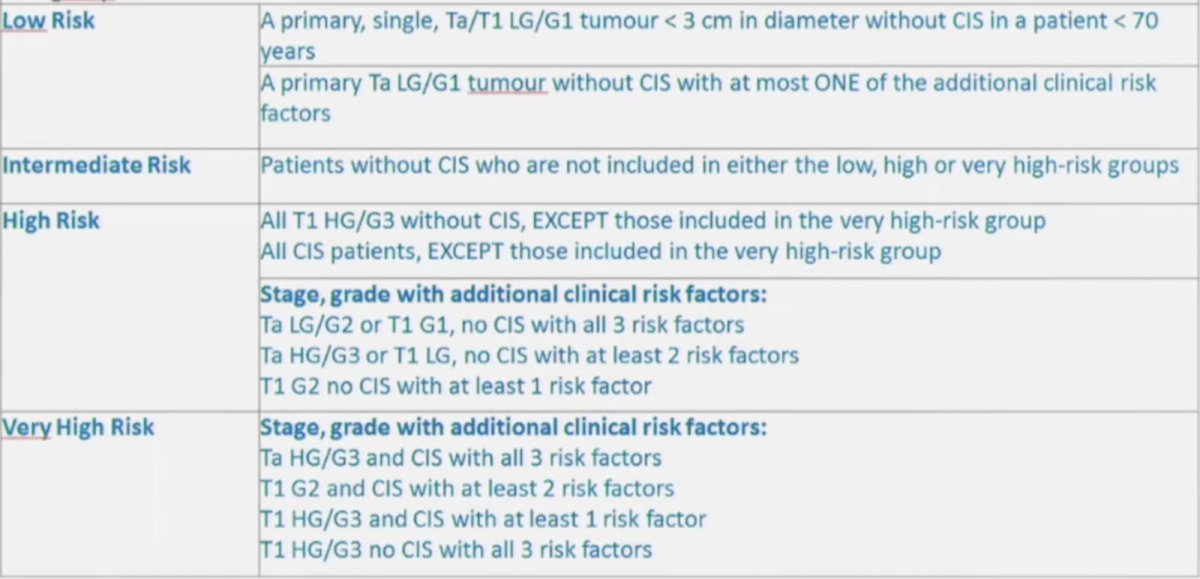
(UroToday.com) The Société Internationale D’Urologie (SIU) 2021 annual meeting included a master class on biomarkers in cancer and a presentation by Dr. Murat Bozlu discussing immune-related biomarkers of bladder cancer. Dr. Bozlu started his presentation by noting that bladder cancer makes up 7% of new cancer cases amongst males, accounting for 4% of cancer mortalities. However, screening for bladder cancer in asymptomatic adults is given a grade I recommendation by the USPSTF (neither a recommendation for or against screening), stating that the current evidence is insufficient to assess the balance of benefits and harms of screening for bladder cancer among asymptomatic adults. Based on clinicopathologic features, the EAU guidelines provide risk group stratification in order to assist with treatment and patient counselling for risk of recurrence and progression:
With regards to bladder cancer biomarkers, the ideal biomarker is: (i) easy to use, (ii) affordable, (iii) reliable and repeatable, (iv) has high specificity, and (v) has high sensitivity. A summary of biomarker utilization is as follows:

Urine cytology is commonly used and has high sensitivity for high-grade tumors (84%), low sensitivity for low-grade tumors (16%), and has a sensitivity of 28-100% for detection of carcinoma in situ. However, interpretation of cytology is user-dependent, and a positive voided cytology can indicate a urothelial tumor anywhere in the urinary tract. A standardized reporting system was implemented in 2016 for reporting urinary cytology, including:
- Adequacy of urine specimens (Adequacy)
- Negative for high-grade urothelial carcinoma (Negative)
- Atypical urothelial cells (AUC)
- Suspicious for high-grade urothelial carcinoma (Suspicious)
- High-grade urothelial carcinoma (HGUC)
- Low-grade urothelial neoplasia (LGUN)
Several biomarkers for diagnosis and follow-up are available, including NMP-22 Protein Test, BTA Stat and BTA TRAK, UroVysion (FISH), ImmunoCyt/uCyt+, Uromonitor and Uromonitor-V2, UroSEEK, EpiCheck, as well as a ‘other biomarkers’ such as TERTp mutations and hypermethylation, miRNA, lncRNAs, and extracellular vesicles. Dr. Bozlu also noted that there are several predictive biomarkers for therapy, such as the cytokine panel CyPRIT, IL-2, IL-8, IL-6, IL-Ira, IL-18, IL-12 (p70), IL-12 (p40, tumor necrosis factor-related apoptosis-inducing ligand, and tumor necrosis factor-beta.
Dr. Bozlu then discussed several of the available biomarkers. Nuclear Matrix Protein 22 (NMP22) is a common protein found in the nucleus of all cells, with bladder cancer cells releasing NMP22 into the urine. The difficulty with this biomarker is that false positives are common, which can be secondary to inflammation/infection, urinary stone disease, foreign bodies, bowel urinary diversion, other urogenital tumors, and instrumentation. BTA-Stat and BTA-TRAK detect human complement factor H-related protein, with BTA stat being a qualitative test and BTA-TRAK being a quantitative ELISA test. Immunocytology is a combination of cytology with immunofluorescence, specifically uCyt+/ImmunoCyt is a combination of three fluorescent monoclonal antibodies: 19A211, M344, and LDQ10. However, false positives are possible with this biomarker secondary to BPH and cystitis. UroVysion includes fluorescent in situ hybridization of chromosomes 3, 7, 17, and 9p21 centromere, specifically highlighting polysomy of 9p21.
Dr. Bozlu emphasizes that unfortunately, most molecular biomarkers have failed to reproduce enough sensitivity and specificity to reliably replace the current mainstay of cystoscopy for investigating bladder cancer. From a prognostic point of view, new molecular markers have yet to be established as reliable indicators of tumor aggressiveness. Furthermore, tumor stage and grade are the current cornerstone factors in establishing prognosis and planning treatment strategies.
CyPRIT is a cytokine panel for response to intravesical therapy, first described by Kamat et al.1 In this study, urine samples were collected for cytokine assay at baseline, immediately before and after BCG instillation at 6 weeks, and immediately before and after the third BCG instillation of the first maintenance course. Levels of 12 cytokines were measured, and changes from baseline were calculated after treatment. Among 130 patients enrolled in this study, increases in single cytokines correlated with recurrence, but the best predictor of recurrence was changes in a combination of cytokines. A nomogram (CyPRIT) constructed using urinary levels of nine inducible cytokines (IL-2, IL-6, IL-8, IL-18, IL-1ra, TRAIL, IFN-γ, IL-12[p70], and TNF-α) predicted the likelihood of recurrence with 85.5% accuracy (95% CI 77.9–93.1%).
With regards to predicting response to intravesical BCG, Dr. Bozlu notes that clinicopathologic features are prognostic as well as predictive for BGC response. However, efforts at using single biomarkers to predict BCG response are inherently compromised due to BCG’s multifaceted mechanism of action. Thus, the best predictors of response to intravesical immunotherapy currently available are grade and stage of tumors, recurrence pattern of prior tumors, nomograms such as the EORTC and CUETO tables, panels of urinary cytokines, and FISH patterns of urine examination.
Dr. Bozlu concluded his presentation with several take-home messages:
- No biomarkers have been accepted for diagnosis or follow-up in routine clinical practice or clinical guidelines
- If the aim is to avoid unnecessary cystoscopy, rather than looking for markers with a high sensitivity and specificity, the focus should be on identifying a marker with a very high negative predictive value
Presented by: Murat Bozlu, MD, Department of Urology, Mersin University School of Medicine, Mersin, Turkey
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Société Internationale D’Urologie (SIU) Hybrid Annual Meeting, Wed, Nov 10 – Sun, Nov 14, 2021.
References:
- Kamat AM, Briggman J, Urbauer DL, et al. Cytokine panel for response to intravesical therapy (CyPRIT): Nomogram of changes in urinary cytokine levels predicts patient response to Bacillus Calmette-Guerin. Eur Urol. 2016 Feb;69(2):197-200.


