(UroToday.com) The Société Internationale D’Urologie (SIU) 2021 annual meeting included a master class on biomarkers in cancer and a presentation by Dr. Wassim Kassouf discussing DDR mutations and response to chemotherapy. For context, Dr. Kassouf noted that for metastatic muscle-invasive bladder cancer, 25% of patients do not response to first-line cisplatin-based chemotherapy, and even among responders the majority will subsequently relapse. For patients with localized muscle-invasive disease, neoadjuvant chemotherapy + radical cystectomy is the standard of care, however, the majority of these patients do not benefit from neoadjuvant chemotherapy. Additionally, this is the cohort of patients that may benefit from an assessment of bladder preservation.
Work from Kukreja et al.1 highlights that the absence of tumor on repeat transurethral resection does not predict the final pathology of T0 at the time of radical cystectomy. Among 1,897 patients treated with radical cystectomy, 157 of these patients were cT0 at the time of TURBT, however, 26% had >= pT2 disease at the time of radical cystectomy. Thus, complete TURBT does not predict pT0 at radical cystectomy, and these results must be taken into context when considering patients for bladder preservation strategies.
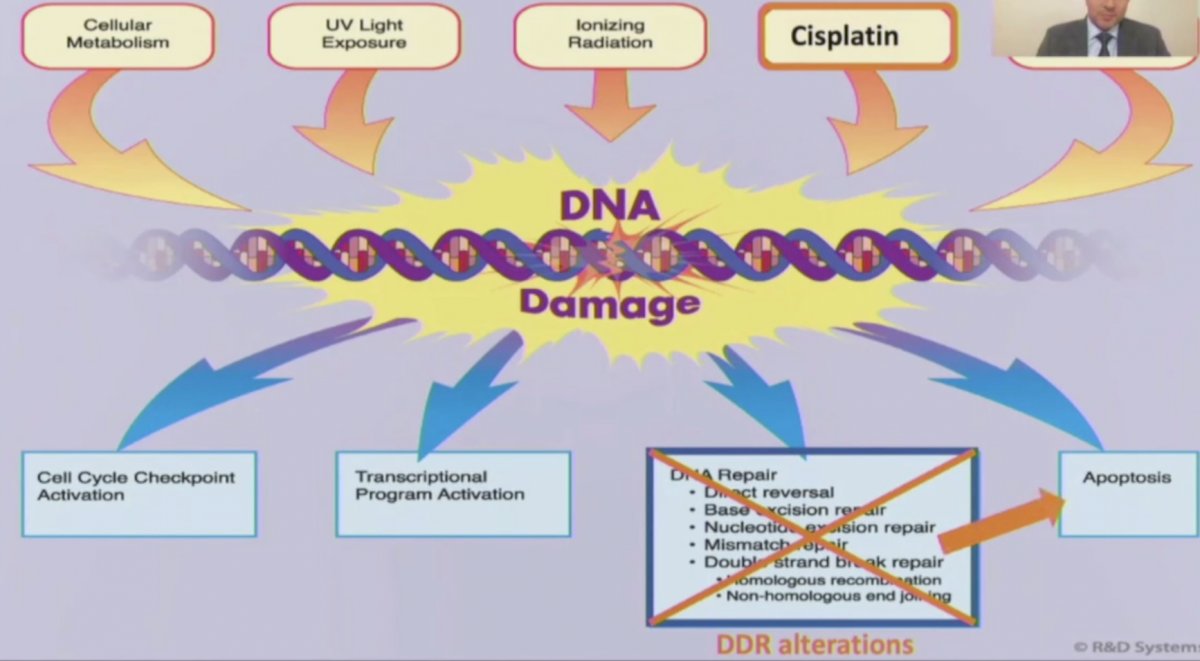
Dr. Kassouf highlighted that there are several mechanisms for inducing DNA damage, including cisplatin-based chemotherapy, which may then lead to DDR alterations and subsequently apoptosis, as summarized in the following figure:
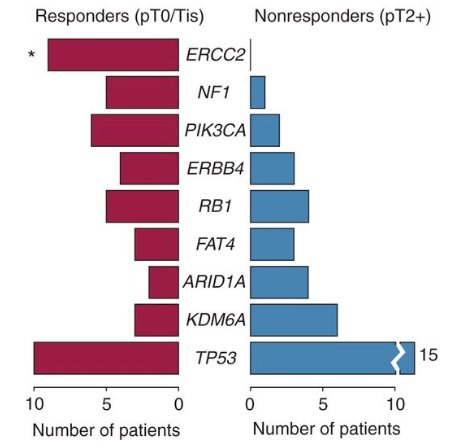
Not all DDR gene alterations are created equal. Previous work from Van Allen et al.2 showed that somatic ERCC2 mutations are associated with pathologic complete response following cisplatin-based neoadjuvant chemotherapy. In this study of 50 patients that had whole-exome sequencing on pretreatment tumor and germline DNA, ERCC2, a nucleotide excision repair gene, was the only significantly mutated gene enriched in the cisplatin responders compared with non-responders:
Follow-up work from Liu et al.3 showed that somatic ERCC2 mutations were associated with improved survival following cisplatin-based neoadjuvant chemotherapy. Among 48 patients, 10 patients had ERCC2 genetic alterations, which was associated with response to therapy: 8 of 20 responders (40%) and 2 of 28 non-responders (7%) had a ERCC2 genetic alteration (OR 8.3, 95% CI 1.4-91.4). Ultimately, 17 patients had disease progression and 15 died, with a statistically significant difference in overall survival among patients with ERCC2 alterations in both the validation cohort and discovery cohort:

It is important to note that association does not equal causality. Specifically, what is the functional consequence of the observed mutation? For example, how does the specific mutation impact the DDR pathway activity? Is the presence of the mutation in the tumor sufficient to drive clinical sensitivity to a specific therapeutic agent?
In 2019, Li et al.4 showed that ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity. In this study, the investigators used a functional assay to test the nucleotide excision repair capacity of clinically observed ERCC2 mutations and found that most ERCC2 helicase domain mutations cannot support nucleotide excision repair. Furthermore, introducing an ERCC2 mutation into a bladder cancer cell line abrogates nucleotide excision repair activity and is sufficient to drive cisplatin sensitivity in an orthotopic xenograft model.
In a recent study from Taber et al.,5 they used integrated multi-omic analysis to assess molecular correlates of cisplatin-based chemotherapy response among 300 patients with muscle-invasive bladder cancer treated with first-line neoadjuvant chemotherapy. They found that DNA-based association of responses converge on genomic instability driven by a high number of chromosomal alterations, indels, and signature 5 mutations and/or BRCA2 mutations. Interestingly, there was no association with ERCC2 mutated tumors and response to chemotherapy.
Dr. Kassouf notes that there are three trials evaluating the performance of DDR alterations in predicting response to cisplatin-based neoadjuvant chemotherapy and subsequent bladder preservation. First, the RETAIN interim results were first presented at GU ASCO 2021, a phase II, multi-institutional clinical trial (NCT02710734) evaluating a risk-adapted approach to the treatment of muscle-invasive bladder cancer. Following neoadjuvant chemotherapy, patients who had at least one mutation and no clinical evidence of disease by restaging TUR and imaging began a pre-defined active surveillance regime. The remaining patients who did not meet these criteria underwent bladder-directed therapy: intravesical therapy (< cT2 post-neoadjuvant chemotherapy), chemoradiation or cystectomy. The trial schema is as follows:
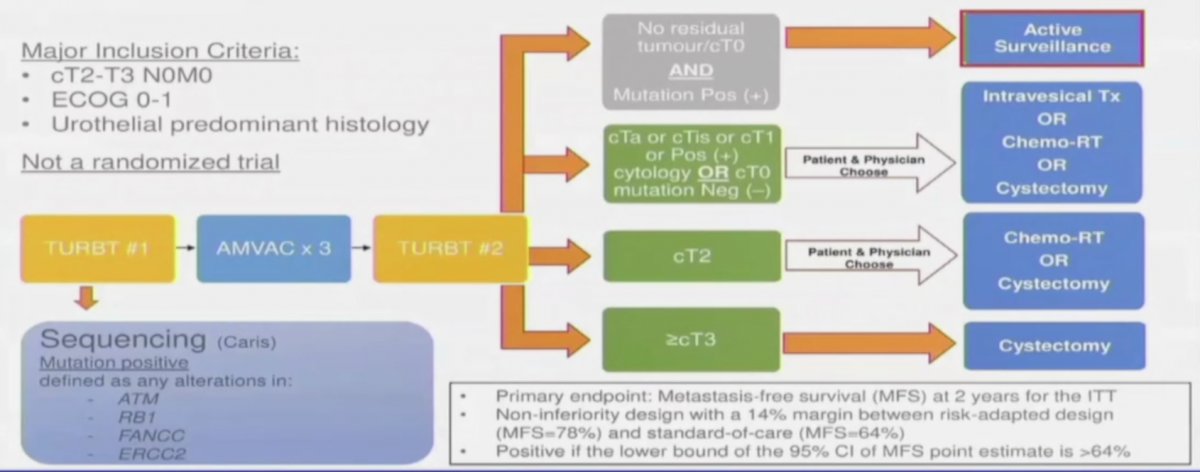
The authors enrolled 71 patients in the intention-to-treat population over 33 months at four academic centers. As of a data cut-off of September 11, 2020, for the ITT patients, 32 have undergone radical cystectomy, 5 underwent chemoradiation and 7 underwent intravesical therapy.
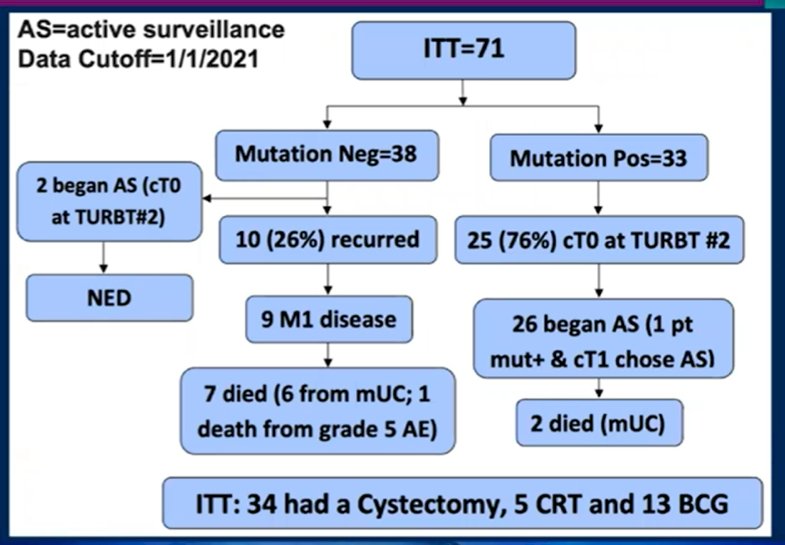
Among the enrolled patients, 33 patients (46%) had a mutation of interest and 28 patients (39%) started active surveillance. 76% of those with a mutation had no residual disease (cT0) at the time of post-neoadjuvant chemotherapy TURBT. With a median follow-up of 14.9 months (range: 3.1-35.3 months), 14 patients who underwent active surveillance had evidence of recurrence (50%), of which two recurred with locally advanced or metastatic disease and have died, 5 recurred with MIBC with one eventual metastatic recurrence, and 7 recurred with NMIBC. Out of the 40 patients who did not undergo upfront radical cystectomy, 3 patients (7.5%) all of whom underwent active surveillance eventually underwent radical cystectomy. Overall, 55% of patients in the ITT cohort and 89% of patients in the active surveillance group had successful bladder preservation. Based on these results, the conclusions were that a risk adapted approach allows active surveillance of a subset of patients with MIBC who achieve pathologic complete response from neoadjuvant chemotherapy, thus sparing the toxicity of consolidative therapy.
The second trial discussed by Dr. Kassouf was the HCRN GU 16-257 trial, first presented at ASCO 2021, assessing gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle-invasive bladder cancer. Patients received 4 cycles of gemcitabine, cisplatin, plus nivolumab followed by clinical restaging including urine cytology, MRI/CT of the bladder, cystoscopy and bladder/prostatic urethral biopsies. Patients achieving a clinical complete response (normal cytology, imaging, and cT0/Ta) were eligible to proceed without cystectomy and receive nivolumab q2 weeks x 8 followed by surveillance; otherwise, patients underwent cystectomy. There were 76 patients enrolled at 7 sites and 64 (84%) patients have completed post-cycle 4 restaging. Thirty-one of 64 patients achieved a clinical complete response (48%; 95% CI 36%-61%), over a median follow-up for clinical complete response patients of 13.7 months (range: 2.5-24 months). The current disposition of the patients in HCRN GU 16-257 is as follows:
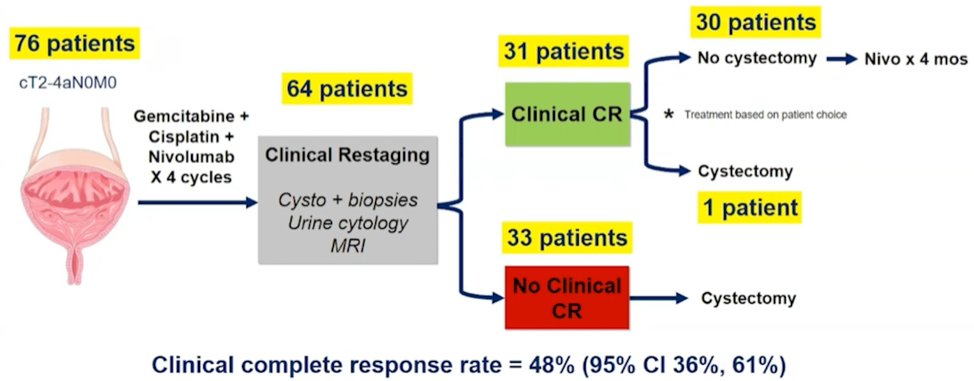
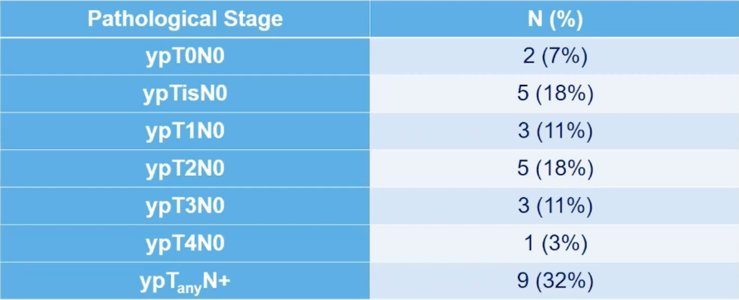
Pathological stage in patients without clinical complete response undergoing immediate cystectomy (n=28) is as follows:
Among 63 patients for which genomic alterations and clinical complete response could be assessed, TMB ≥ 10 mut/Mb (p=0.02) or mutant ERCC2 (p=0.02) were associated with clinical complete response or partial complete response. However, ATM, FANCC, or RB1 alterations were not associated with complete clinical response or partial clinical response.
The third study, A031707 (has not yet reported results), is assessing bladder-sparing following chemotherapy among patients with select DDR gene alterations. Patients with cT2-T4N0 bladder cancer will have genomic sequencing of the TURBT specimen, treatment with investigator choice of neoadjuvant chemotherapy followed by molecular stratification to DDR deleterious alteration versus DDR wild-type. Patients with DDR deletions that are cT0/CIS will undergo bladder preservation, whereas those with >= T1 response to neoadjuvant chemotherapy will have radical cystectomy or chemoradiation. DDR wild-type patients will be treated with radical cystectomy or chemoradiation. The trial schema is as follows:

Dr. Kassouf concluded his presentation of DDR mutations and response to chemotherapy with the following take-home messages:
- DDR alterations are common in muscle-invasive bladder cancer patients
- There is an association with response to cisplatin-based chemotherapy
- There is functional validation of the ERCC2 mutation
- There are inconsistent results with specific DDR mutations and chemotherapy response
- Several ongoing trials are evaluating the clinical utility of DDR alterations in bladder preservation following systemic therapy
- Further evaluation is warranted for assessing a single gene signature versus broader DDR-deficient tumor phenotypes
- The role of combined biomarkers is currently unknown, with respect to DDR alterations + molecular subtyping
Presented by: Wassim Kassouf, MD, CM, FRCSC, Professor, Department of Surgery (Urology), McGill University, Montreal, Quebec, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Société Internationale D’Urologie (SIU) Hybrid Annual Meeting, Wed, Nov 10 – Sun, Nov 14, 2021.
References:
- Kukreja JB, Porten S, Golla V, et al. Absence of tumor on repeat transurethral resection of bladder tumor does not predict final pathologic T0 stage in bladder cancer treated with radical cystectomy. Eur Urol Focus. 2018 Sep;4(5):720-724.
- Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlated with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014 Oct;4(10):1140-1153.
- Liu D, Plimack ER, Hoffman-Censits J, et al. Clinical validation of chemotherapy response biomarker ERCC2 in muscle-invasive urothelial bladder carcinoma. JAMA Oncol. 2016;2(8):1094-1096.
- Li Q, Damish AW, Frazier Z, et al. ERCC2 helicase domain mutations confer nucleotide excision repair deficiency and drive cisplatin sensitivity in muscle-invasive bladder cancer. Clin Cancer Res. 2019 Feb 1;25(3):977-988.
- Taber A, Christensen E, Lamy P, et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat Commun 2020;11:4858.


