(UroToday.com) The Société Internationale D’Urologie (SIU) 2021 annual meeting included a master class on biomarkers in cancer and a presentation by Dr. Sia Daneshmand discussing serum biomarkers in invasive bladder cancer. Dr. Daneshmand started by emphasizing that unfortunately, up to 50% of patients after radical cystectomy experience disease recurrence within five years of surgery. Furthermore, there are no currently available tests to determine which patients will have residual disease or be at risk of relapse. There are several options for liquid biopsies in urothelial carcinoma of the bladder and upper tract urothelial carcinoma as highlighted in the following figure:

Currently, there is an unmet need for novel markers, including (i) minimally invasive approaches to detecting residual disease after surgery, (ii) stratifying high-risk patients with regards to the need for adjuvant therapy versus surveillance, and (iii) monitoring disease relapse at earlier stages.
The role of Ki-67, MRE11, and PD-L1 have recently been evaluated as predictive biomarkers for recurrence in patients with muscle-invasive bladder cancer. In work from Dr. Daneshmand’s group, Fossum et al.1 queried the USC cystectomy database, collecting clinicopathological data developing a tissue microarray among 42 patients, analyzing for the presence of Ki-67, MRE11, and PD-L1 using immunofluorescence and immunohistochemistry. Compared to normal bladder tissue, tumors had increased expression of Ki-67 (p<0.01) and PD-L1 (p<0.05). High Ki-67 was associated with recurrence pattern (local versus distant), and Ki-67 cell density varied by recurrence type: local recurrence (1354 cells/mm2), distant recurrence (557 cells/mm2) and no recurrence (1111 cells/mm2) (p=0.034).
Dr. Daneshmand’s group has also assessed the association between precystectomy epithelial tumor marker response to neoadjuvant chemotherapy and oncologic outcomes.2 Specifically, they assessed serum levels of Carbohydrate Antigen 125 (CA-125), Carbohydrate Antigen 19-9 (CA 19-9), and Carcinoembryonic Antigen (CEA) in 387 patients with invasive bladder cancer. They found that elevated pre-cystectomy level of any tumor marker (31% of patients) was independently associated with worse recurrence-free survival (HR 2.81; p < 0.001) and overall survival (HR 3.97; p < 0.001). There were 125 (37%) patients that underwent neoadjuvant chemotherapy, of whom 59 had a complete tumor marker profile and 30 (51%) had an elevated pre-neoadjuvant chemotherapy tumor marker. Following completion of chemotherapy, 10/30 (33%) patients normalized their tumor markers, while 20/30 (67%) had one or more persistently elevated markers. Between these groups, there was no difference in clinical or pathological stage (p = 0.54 and p = 0.09, respectively).
In an updated analysis assessing CA-125, CA 19-9 and CEA presented at the 2021 GU ASCO meeting, among 199 patients, 63 had both pre-and post-neoadjuvant chemotherapy tumor markers. Patients with elevated pre-neoadjuvant chemotherapy tumor markers had significantly higher rate of pathologic upstaging compared to those with normal pre-neoadjuvant chemotherapy tumor markers (62% vs. 22.5%, respectively; p < 0.001). Compared to tumor marker responders, tumor marker non-responders had a significantly higher rate of recurrence (70% vs 34%) and shorter median time to recurrence (4.2 months vs 13.5 months) (p = 0.03).
Dr. Daneshmand for the remainder of his presentation discussed the utility of circulating tumor DNA (ctDNA). In 2017, Vandekerkhove et al.3 applied a combination of whole-exome sequencing and targeted sequencing across 50 bladder cancer driver genes to plasma cell-free DNA (cfDNA) from 51 patients with aggressive bladder cancer, including 37 with metastatic disease. They found that the majority of patients with metastasis (only 14% of patients with localized disease) had ctDNA proportions above 2% of total cfDNA (median 16.5%, range 3.9%-72.6%). Furthermore, there was an aggressive mutational landscape in metastatic bladder cancer with 95% of patients harboring deleterious alterations to TP53, RB1, or MDM2, and 70% harboring a mutation or disrupting rearrangement affecting chromatin modifiers such as ARID1A. As such, these investigators concluded that liquid biopsy can inform valuable context to better dissect therapy response and accelerate rational implementation of precision oncology.
ctDNA has also been shown to be a biomarker for early detection of metastatic relapse. Among 68 patients with locally advanced bladder cancer Christensen et al.4 noted that the presence of ctDNA was highly prognostic at diagnosis before chemotherapy (HR 29.1; p = 0.001). There were three time points of significant interest. First, ctDNA status at diagnosis before chemotherapy was strongly prognostic:
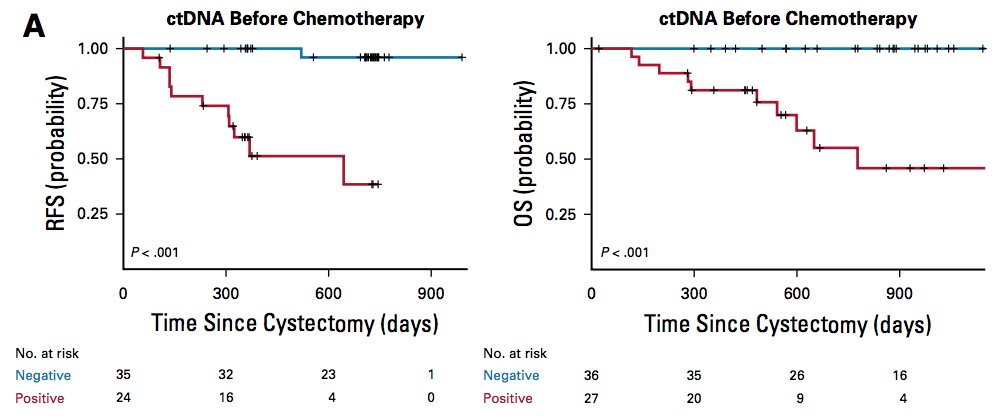
Second, after chemotherapy and before cystectomy was also prognostic of patient outcome:
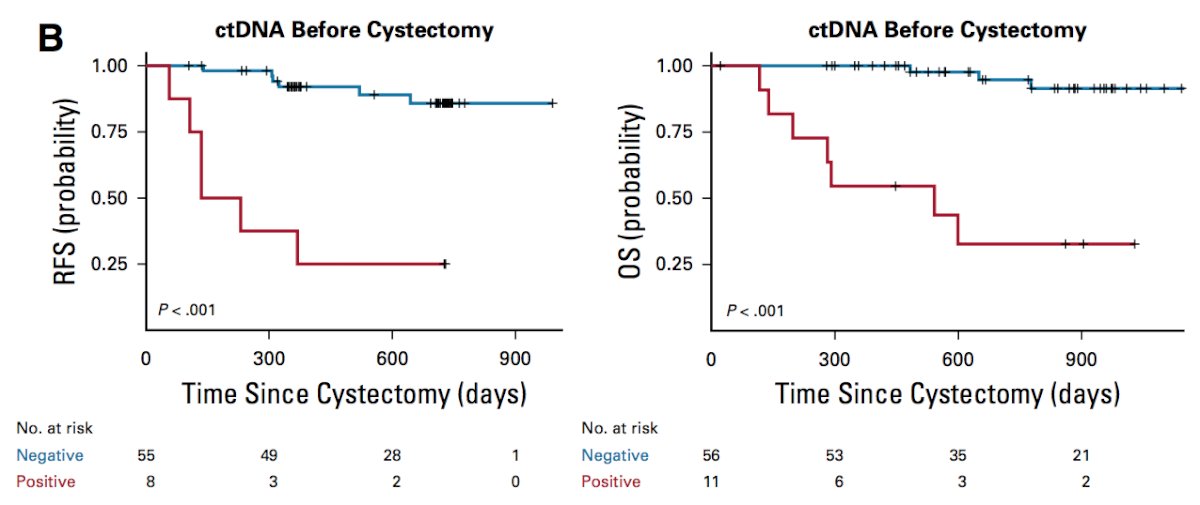
Third, and arguably most significantly, plasma ctDNA status during disease surveillance after cystectomy was highly prognostic:
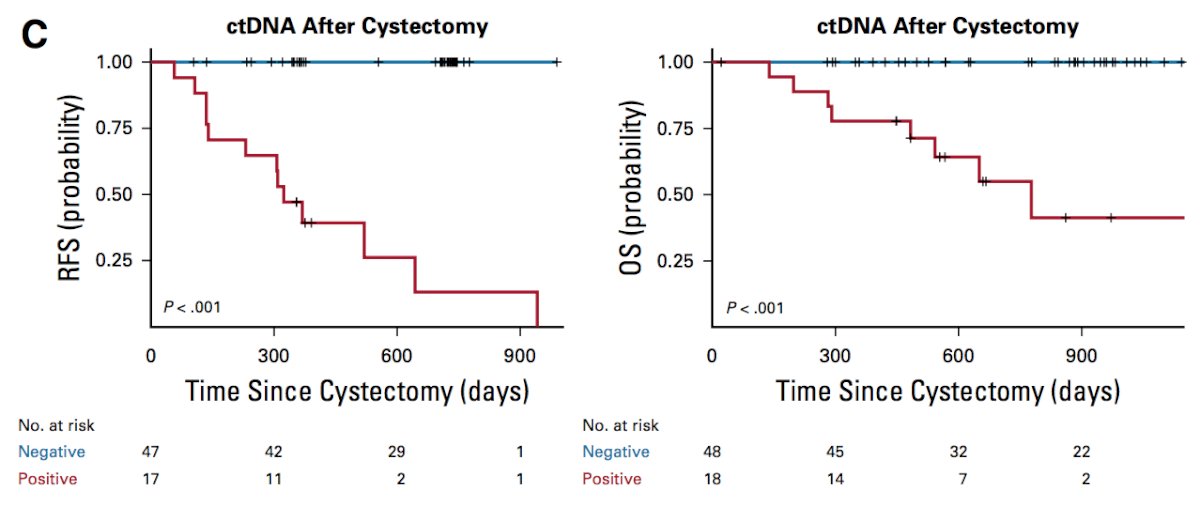
Finally, 100% of pre-cystectomy ctDNA-positive patients had residual tumor (stage >= T1) and/or lymph node metastasis at the time of cystectomy, and 100% of patients (36 of 36) with pT0 at cystectomy were ctDNA negative.
Although the data is promising, biomarkers are not currently recommended by the guidelines. Specifically, the EAU guidelines state that “there is insufficient evidence to use tumor mutational burden, molecular subtypes, immune or other gene expression signatures, for the management of patients with urothelial carcinoma.” The potential role for biomarkers in patients with bladder cancer includes (i) identifying NMIBC patients at risk of progression, (ii) identifying disease response to systemic therapy, (iii) risk stratification and prognostication of disease recurrence pre- and post-radical cystectomy, (iv) predicting patients at risk of recurrence after cystectomy, and (v) identifying patients suited for salvage therapies sooner.
Dr. Daneshmand concluded his presentation of serum biomarkers in muscle-invasive bladder cancer with the following conclusions:
- Biomarkers in urothelial carcinoma patients are very promising
- They provide early risk stratification of patients, prediction of treatment response, and early detection of metastatic disease
- They may help identify patients at highest risk for disease progression in NMIBC and muscle-invasive bladder cancer
- We need to determine which patients will benefit from neoadjuvant chemotherapy
- Precision medicine is likely in the near future, with molecular characterization and personalized treatment approaches
Presented by: Sia Daneshmand, MD, Professor of Urology (Clinical Scholar), Director of Urologic Oncology, Director of Clinical Research, University of Southern California, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Société Internationale D’Urologie (SIU) Hybrid Annual Meeting, Wed, Nov 10 – Sun, Nov 14, 2021.
References:
- Fossum CC, Xiong Y, Magliocco A, et al. Role of Ki-67, MRE11, and PD-L1 as Predictive Biomarkers for Recurrence Pattern in Muscle-invasive Bladder Cancer. Anticancer Res. 2021 Aug;41(8):3851-3857.
- Bazargani ST, Clifford TG, Djaladat H, et al. Association between precystectomy epithelial tumor marker response to neoadjuvant chemotherapy and oncologic outcomes in urothelial bladder cancer. Urol Oncol. 2019 Jan;37(1):1-11.
- Vandekerkhove G, Todenhofer T, Annala M, et al. Circulating tumor DNA reveals clinically actionable somatic genome of metastatic bladder cancer. Clin Cancer Res. 2017 Nov 1;23(21):6487-6497.
- Christensen E, Birkenkamp-Demtroder K, Sethi H, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol. 2019 Jun 20;37(18):1547-1557.


