(UroToday.com) The Société Internationale D’Urologie (SIU) 2021 annual meeting included a master class on biomarkers in cancer and a presentation by Dr. Seth Lerner discussing molecular subtypes of bladder cancer. Dr. Lerner started by highlighting seminal work from his group that used the bladder cancer TCGA dataset to provide a comprehensive molecular characterization of muscle invasive bladder cancer.1 Among 412 muscle-invasive bladder cancer specimens, this work identified five expression subtypes including: luminal, luminal papillary, luminal infiltrated, basal squamous, and neuronal,
Furthermore, these subtypes are prognostic, with the best survival among patients with luminal-papillary tumors, and the worst prognosis for neuronal tumors:
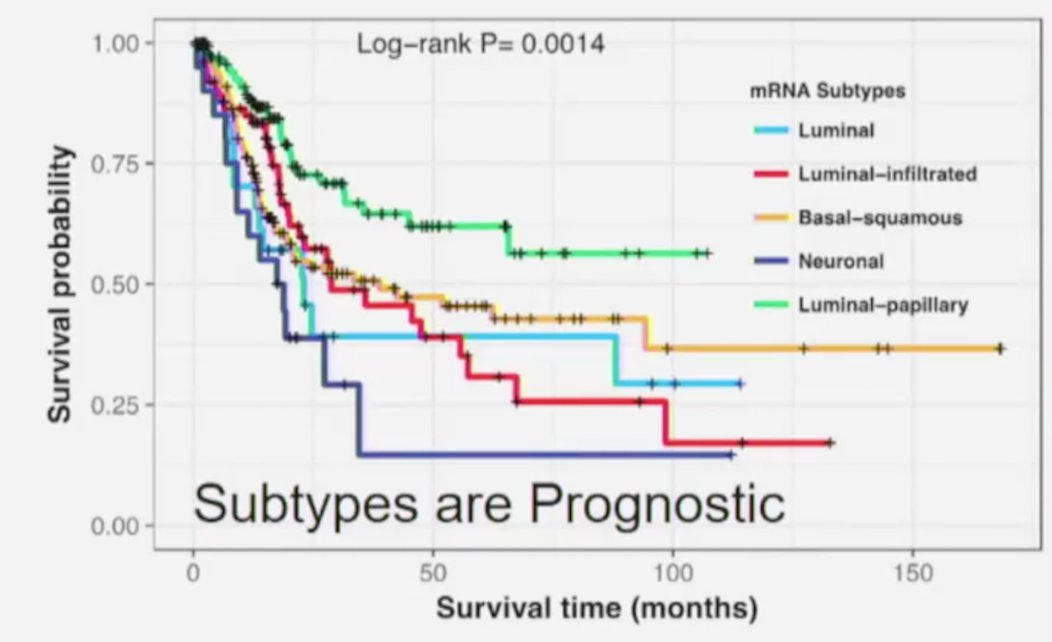
Subsequent work from Seiler et al.2 investigated the ability of molecular subtypes to predict pathological downstaging and survival after neoadjuvant chemotherapy. Among 343 patients with muscle-invasive bladder cancer that had whole transcriptome profiling performed pre-neoadjuvant chemotherapy, a single-sample genomic subtyping classifier was developed to predict consensus subtypes with highest clinical impact in the context of neoadjuvant chemotherapy. They found that the genomic subtyping classifier was able to predict four consensus molecular subtypes (claudin-low, basal, luminal-infiltrated, and luminal) with high accuracy (73%). Furthermore, the following findings were highlighted in this study:
- Luminal tumors had the best overall survival with and without neoadjuvant chemotherapy
- Claudin-low tumors were associated with poor overall survival irrespective of treatment regimen
- Basal tumors showed the most improvement in overall survival with neoadjuvant chemotherapy compared with surgery alone
In 2019, Dr. Lerner’s group published their work looking at the TCGA subtypes and response to checkpoint inhibition.3 This study showed that the TCGA subtypes were also prognostic for response to checkpoint inhibitor therapy, noting that the survival probability was extraordinarily high for the neuronal subtype:
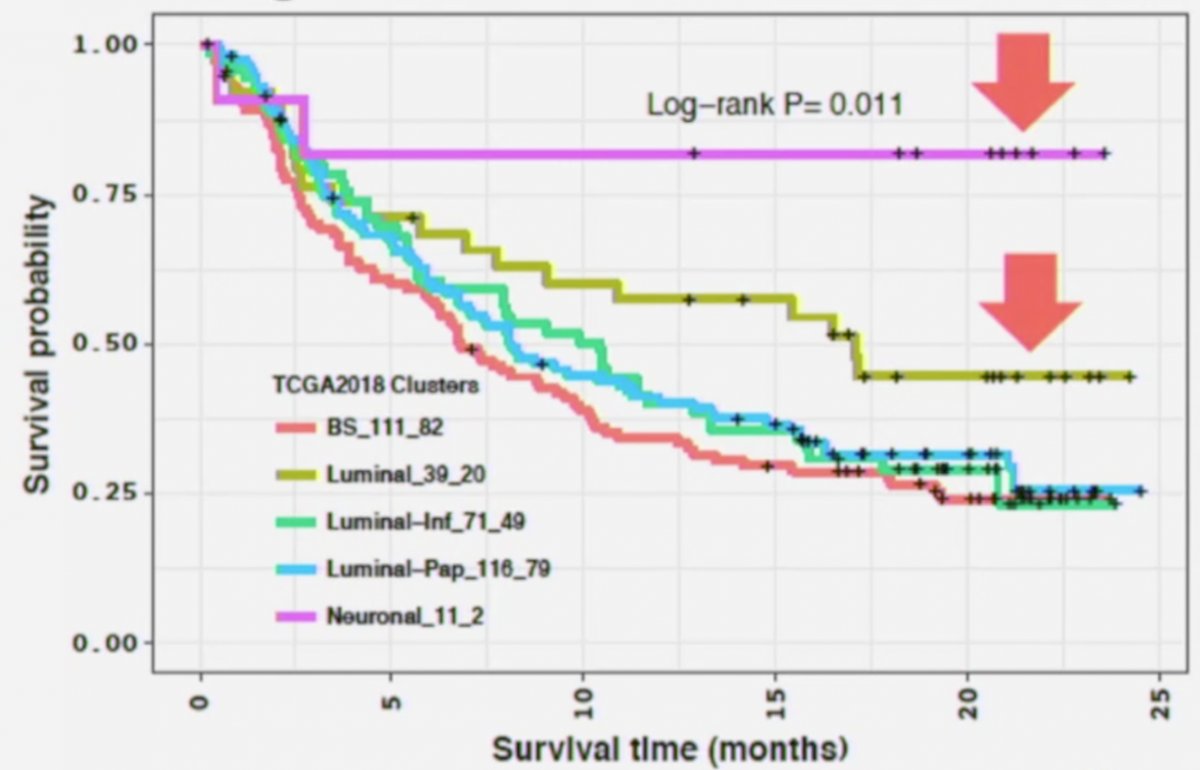
Notably, none of the neuronal tumors were immune inflamed, 8/11 (77%) were immune excluded, there was no major role for CD8+ T-effector cells, neuronal tumors had the lowest TGFB1 and TGFBR1 expression, and were associated with p53 mutation (11/11) and RTB1 loss (7/11).
Two trials have assessed the utility of biomarker analysis in the neoadjuvant immune checkpoint inhibitor disease space, specifically the ABACUS4 and PURE-015 trials. In the ABACUS trial testing atezolizumab, baseline biomarkers showed that the presence of preexisting activated T cells was more prominent than expected and correlated with outcome. Responding tumors showed a predominant expression of genes related to tissue repair after treatment, making tumor biomarker interpretation challenging in this group. Additionally, stromal factors such as TGF-β and fibroblast activation protein were linked to resistance, as was high expression of cell cycle gene signatures after treatment. In the PURE-1 trial assessing pembrolizumab, the neuroendocrine-like subtype had the worst 2-year progression-free survival (33%) and the genomic subtyping classifier claudin-low subtype had the best, with no recurrences at 2 years. Basal subtypes with higher Immune190 scores showed 100% 2-year progression-free survival after pembrolizumab therapy (p = 0.04, compared with basal-Immune190 low).
Dr. Lerner then highlighted a 2020 publication from Kamoun and colleagues6 in European Urology which described a consensus molecular classification of muscle-invasive bladder cancer. This approach described 6 consensus subtypes as follows:
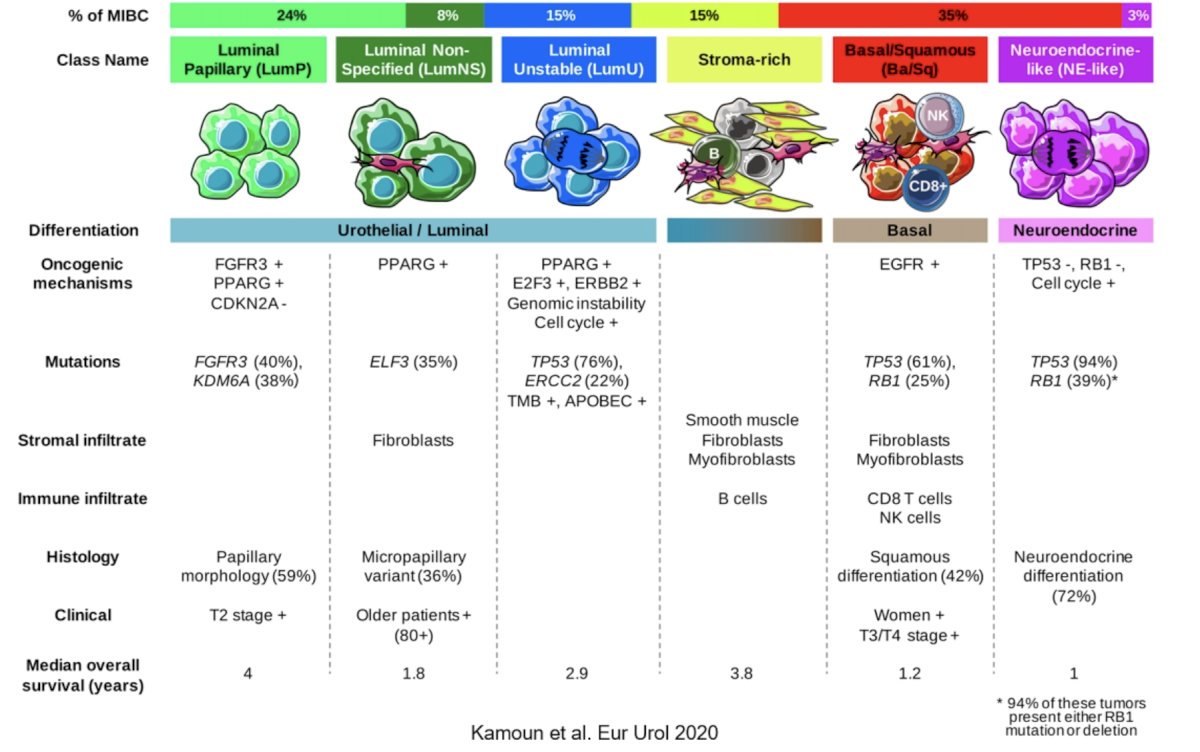
Ultimately, patients with luminal papillary tumors represent nearly a quarter of all patients and have the best survival while basal/squamous tumors represent nearly a third of patients and have a relatively poor prognosis but may derive the greatest benefit from neoadjuvant chemotherapy.
Dr. Lerner and colleagues are designing a subtyping-based clinical trial as highlighted by the following schema:

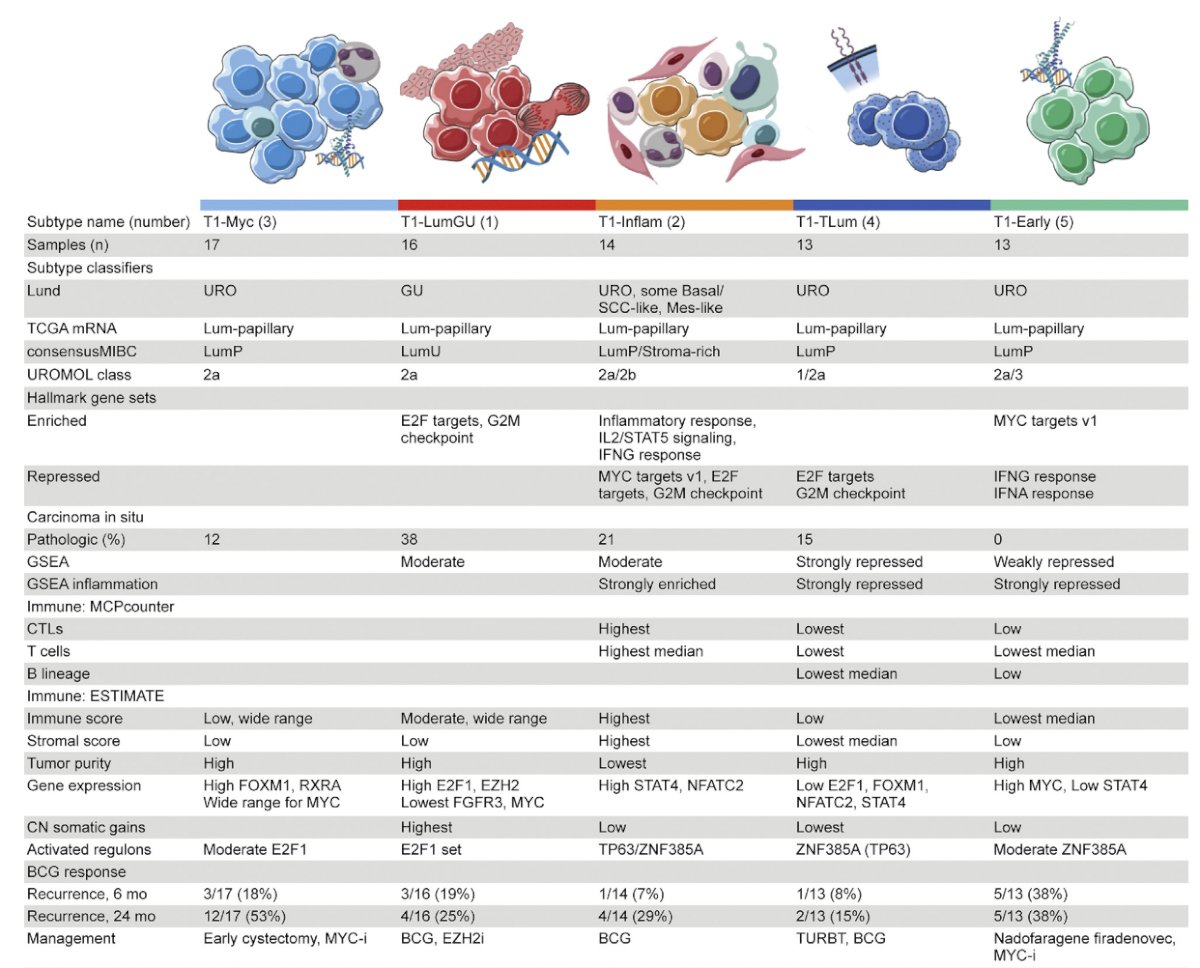
Subtyping has also recently been incorporated into high-grade T1 tumors, specifically work from Dr. Josh Meeks’ group.7 These investigators performed transcriptome profiling and unsupervised clustering, identifying five consensus subtypes of T1 tumors treated with repeat transurethral resection and induction and maintenance BCG. Additionally, findings were as follows and highlighted in the subsequent table:
- T1-LumGU subtype: associated with carcinoma in situ, had high E2F1 and EZH2 expression and was enriched in E2F target and G2M checkpoint hallmarks
- T1-Inflam subtype: was inflamed and infiltrated with immune cells
- T1-TLum subtype: had the highest median luminal papillary score and FGFR3 expression, no recurrence events, and the fewest copy number gains
- T1-Myc and T1-Early subtypes: had the most recurrences (14/30 within 24 months) and the highest median MYC expression
- T1-Early subtype: had five (38%) recurrences within the first 6 months of BCG, and repressed IFN-α and IFN-γ hallmarks and inflammation
Finally, Lindskrog et al.8 published recent work in Nature Communications, performing an integrative multi-omics analysis of 834 patients diagnosed with NMIBC. Among 613 Ta, 238 T1, and 11 CIS patients, transcriptomic analysis identified four classes (1, 2a, 2b, and 3) of tumors reflecting tumor biology and disease aggressiveness. High chromosomal instability, p53-pathway disruption, and APOBEC-related mutations were significantly associated with transcriptomic class 2a and poor outcomes. RNA-derived immune cell infiltration is associated with chromosomally unstable tumors and enriched in class 2b. Additionally, spatial proteomics analysis confirms the higher infiltration of class 2b tumors and demonstrates an association between higher immune cell infiltration and lower recurrence rates. As follows is the summary characteristics of each class from this study:

Dr. Lerner concluded his presentation of molecular subtypes of bladder cancer with the following take-home messages:
- RNA expression-based subtypes in muscle-invasive bladder cancer and non-muscle invasive bladder cancer are unique and offer insights to biology and subtype specific treatment
- Post neoadjuvant chemotherapy and immunotherapy response is associated with a scar-like subtype and a better prognosis and may provide insights into the mechanisms of treatment resistance
- Clinical trials validating predictive biomarkers are essential for precision medicine
Presented by: Seth Lerner, MD, FACS, Beth and Dave Swalm Chair in Urologic Oncology, Scott Department of Urology, Baylor College of Medicine, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Société Internationale D’Urologie (SIU) Hybrid Annual Meeting, Wed, Nov 10 – Sun, Nov 14, 2021.
References:
- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 2017;171(3):540-556.
- Seiler R, Al Deen Ashab H, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017 Oct;72(4):544-554.
- Kim J, Kwiatkowski D, McConkey DJ, et al. The Cancer Genome Atlas Expression Subtypes Stratify Response to Checkpoint Inhibition in Advanced Urothelial Cancer and Identify a Subset of Patients with High Survival Probability. Eur Urol. 2019 Jun;75(6):961-964.
- Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019;25:1706-1714.
- Necchi A, Raggi D, Gallina A, et al. Impact of molecular subtyping and immune infiltration on pathological response and outcome following neoadjuvant pembrolizumab in muscle-invasive bladder cancer. Eur Urol. 2020 Jun;77(6):701-710.
- Kamoun A, de Reynies A, Allory Y, et al. A Consensus Molecular Classification of Muscle-invasive bladder cancer. Eur Urol. 2020 Apr;77(4):420-433.
- Robertson AG, Groeneveld CS, Jordan B, et al. Identification of Differential Tumor Subtypes of T1 Bladder Cancer. Eur Urol. 2020 Oct;78(4):533-537.
- Lindskrog SV, Prip F, Lamy P, et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun. 2021 Apr 16;12(1):2301.


