(UroToday.com) At the metastatic castration resistant prostate cancer (mCRPC) session at the Society of Nuclear Medicine & Molecular Imaging 2021 Annual Meeting, Dr. Daniel Lee discussed osseous molecular radiotherapy for mCRPC. Dr. Lee highlighted that mCRPC is the terminal phase of the disease, with a poor prognosis, encompassing many patients with primarily skeletal disease. Indeed, skeletal disease is the major determinant of morbidity and mortality, affecting both hematopoiesis and structural integrity. Radiotherapy in prostate cancer includes Sr-89 (Metastron®), Sm-153 EDTMP (Quadramet®), and radium-223 (Xofigo®).
The pivotal phase III trial of radium-223 in mCRPC (ALSYMPCA) randomly assigned 921 patients, to radium-223 or matching placebo in a 2:1 ratio.1 The primary endpoint of this trial was overall survival, which showed at the interim analysis that radium-223 significantly improved overall survival compared to placebo (14.0 months vs 11.2 months; HR 0.70, 95% CI 0.55-0.88). The updated analysis confirmed that radium-223 survival benefit (median, 14.9 months vs. 11.3 months; hazard ratio, 0.70; 95% CI, 0.58 to 0.83). Hematologic and nonhematologic adverse events occurred in at least 5% of patients in either study group. Overall, no clinically meaningful differences in the frequency of grade 3 or 4 adverse events were observed between the study groups. Grade 3 febrile neutropenia was reported in one patient (<1%) in the radium-223 group and in one patient (<1%) in the placebo group. Only one grade 5 hematologic adverse event was considered to be possibly related to the study drug: thrombocytopenia in a patient in the radium-223 group, who died from pneumonia with hypoxemia, with no evidence of bleeding. For serious adverse events that occurred in at least 5% of patients in the radium-223 group or the placebo group, the respective frequencies were as follows: disease progression (11% and 12%), bone pain (10% and 16%), anemia (8% and 9%), and spinal cord compression (4% and 5%).
Previous studies have suggested that PSA is an unreliable marker for radium-223 response. Keizman et al.2 retrospectively evaluated the computed tomography (CT) and bone scintigraphy response of mCRPC patients treated with radium-223, in eight centers in three countries. Among 130 patients that were included, the majority (n=84, 65%) received radium-223 post docetaxel. In patients with available data, a transient increase in bone metastases-related pain was observed in 27% (n=33/124) and an improvement of bone metastases-related pain on treatment with radium-223 was noted in 49% of patients (n=61/124). An increase in PSA occurred in 80% (n=101 of 127 patients with available data) during radium-223 treatment, whereas 20% (n=26) had a decline in PSA (14%, n=18, had a PSA decline of >= 30%):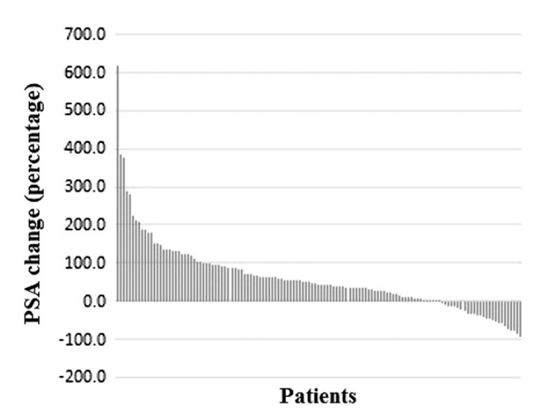
Furthermore, alkaline phosphatase is also not entirely reliable, considering that a decrease in alkaline phosphatase during radium-223 therapy versus the baseline level occurred in 44% of patients. Among patients with baseline alkaline phosphatase, decrease in the level >= 30% during radium-223 therapy was seen in 50% of patients:
Importantly, imaging may be able to predict response to radium-223 with correlation with bone imaging uptake (MDP and NaF-18) and absorbed dose. Furthermore, bone scan index is a quantitative parameter of bone metastasis burden and may be predictive of response.
Currently, there are mixed results of combination regimens with radium-223. Early data from combination with abiraterone was promising, but later data in the ERA 223 trial showed higher rates of skeletal-related events, therefore these agents should not be used together. Early data from combination with enzalutamide was also promising, and in combination with bone health agents such as denosumab or zoledronic acid, the combination appears to be well tolerated. Docetaxel chemotherapy is currently being tested in the phase 3 DORA trial. Overall, combination regimens should only be used in the context of a clinical trial.
How much we can treat with radium-223 has been tested with higher doses, with 250 kBq/kg being well-tolerated in a phase 1 trial. Furthermore, in a phase 2 trial of three dosing regimens, high-dose (88 kBq/kg q4 weeks x 6) was associated with a higher rate of Grade 3 or higher adverse events and discontinuation of participation without significant improvement in outcomes. An extended regimen of 12 injections of standard dose (55 kBq/kg q4 weeks) was also associated with more severe adverse events, discontinuation, and without improved outcomes. Finally, retreatment with a second regimen of six standard dose injections has been reported as well tolerated. It is important to note that radium-223 should not be used in the setting of visceral metastases, concurrent hormonal therapy, chemotherapy, other systemic radionuclide therapy, or hemi-body radiation therapy, or spinal cord compression.
The role of radium-223 in the era of PSMA at this point is probably conjecture, but there is likely a smaller role for radium-223 given that PSMA can also treat soft tissue disease. However, there may be a continuing role in the case of bone only or bone predominant disease and it may continue when used as part of combination regimens. There are many questions that remain unanswered, including earlier use in the course of the disease, combination with other classes of agents, and dosimetry.
Dr. Lee concluded his presentation of the utilization of radium-223 with the following take-home messages:
- Radium-223 is safe and effective, offering improved overall survival and skeletal-related event-free survival
- Hematological effects are the major toxicity
- Outcomes may be better if given earlier in the course of the disease
- Biomarkers may be unreliable for response assessment
- Dosimetry and alternate dosing regimens may be possible
- Combination regimens have deleterious effects with abiraterone and should only be explored in the context of clinical trials
- Radium-223 should not be used in the acute setting, for example with spinal cord compression
- There is likely a smaller role for radium-223 in the era of PSMA theranostics
Presented by: Daniel J. Lee, MD, Ochsner Health System, New Orleans, LA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the Society of Nuclear Medicine & Molecular Imaging - 2021 Virtual Meeting, June 11-15, 2021
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Keizman D, Fosboel MO, Reichegger H, et al. Imaging response during therapy with radium-223 for castration-resistant prostate cancer with bone metastases-analysis of an international multicenter database. Prostate Cancer Prostatic Dis. 2017 Sep;20(3):289-293.


