(UroToday.com) Beginning with the introduction of docetaxel for metastatic castration resistant prostate cancer (mCRPC) in 2004, there has been a dramatic and rapid proliferation of systemic therapy options in advanced prostate cancer including a number of novel hormonal therapies (including abiraterone acetate and enzalutamide), second-line chemotherapy (cabazitaxel), bone-targeting agents (radium-223) and other targeted agents (including olaparib, rucaparib, and pembrolizumab), each of which has proven survival benefits. Radium-223 acts as an alpha-emitting calcium mimetic which targets bony metastatasis. Recently, theranostic treatment with prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy, including most notably 177lutetium-PSMA-617 as well as others, has demonstrated promising activity among pre-treated individuals with mCRPC. Given the similarity of radionuclide activity between 177lutetium-PSMA-617 and radium-223, in the Center for Therapy Excellence Young Investigator Award session at the Society of Nuclear Medicine & Molecular Imaging (SNMMI) 2021 Annual Meeting, Dr. Justus Baumgarten explored the feasibility of immediate onset of 177lutetium-PSMA-617 after radium-223.
To do so, the authors identified 29 patients with progressive metastatic castration resistant prostate cancer who started 177lutetium-PSMA-617 within 5 ± 3 weeks after the last radium-223 administration. Patients further had to have progressive bone disease based on bone scan with evidence of 2 or more new bony lesions or progressive bone marrow involvement. Patients had received a median of 4 (range 3-6) radium-223 injections and then received 4 (2-5) cycles of 177Lu-PSMA, with mean activity per cycle of 6.7 ± 2.1 GBq. The mean cumulative activity of 177Lu-PSMA was 30.7 ± 23.4 GBq.

The mean follow-up with 25 +/- 16 months. Over this time, patients were assessed with 18F-NaF-PET/CT, bone scan, and 68Ga-PSMA PET/CT, according to PROMISE miTNM classification (ever 2-4 cycles) and biochemically with PSA testing every 4 weeks. Further, the authors assessed hematological parameters at baseline, prior to and 2 to 4 weeks after each administration, and throughout follow-up. Toxicity was classified using Common Terminology Criteria for Adverse Events v5.0. The authors examined the correlation between skeletal tumor burden (defined as disseminated/diffuse vs. oligometastatic) and the cumulated activity with the treatment-induced hematotoxicity, using Spearman's rank correlation coefficient. Further, the authors assessed survival using the Kaplan-Meier method.
Among the included patients receiving 177lutetium-PSMA-617 following radium-223, 25 patients had mild (grade 1) and 3 patients moderate (grade 2) bone marrow impairment prior to 177lutetium-PSMA-617. 177lutetium-PSMA-617 induced significant bone marrow toxicity (grade 3-4) necessitating blood transfusion in 5 patients, dose reduction in 2 patients, and led to treatment discontinuation in 7 patients. Further, moderate renal function loss (grade 2) was seen in 7 patients, but high-grade nephrotoxicity was not seen.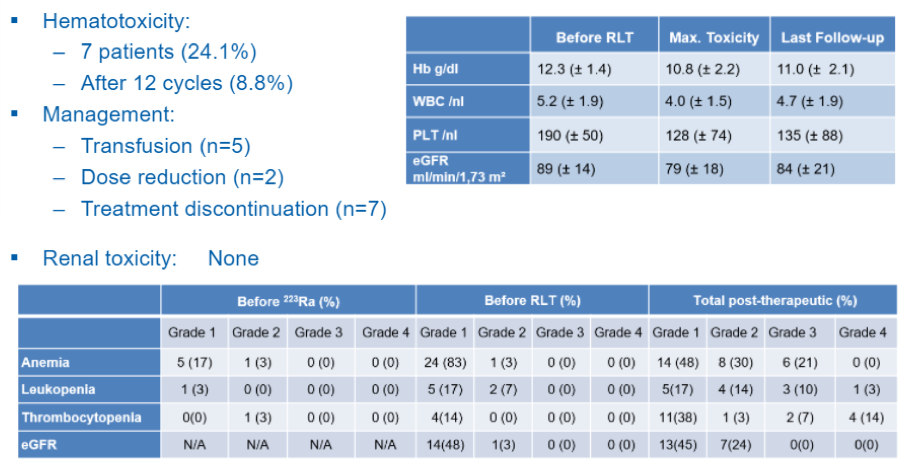
Assessing the response to 177lutetium-PSMA-617, the authors observed partial response in 12 patients (41.4%), stable disease in 8 patients (27.6%), and progressive disease in 9 patients (31.0%). The median progression-free survival and overall survival were 9 months (95 % CI 6-12) and 18 months (95 % CI 11-25), respectively.
Patients who had evidence of disease control had significantly prolonged overall survival: 33 months (95% CI 21-45 months) among those with partial response, 18 months (95% CI 8-28) among those with stable disease, and 11 months (95% CI 2-20) among those with progressive disease. As may be expected, disease burden at baseline (disseminated/diffuse bone involvement, n=12 vs oligometastatic) at baseline was associated with a significantly shorter overall survival (36 vs. 14 months, p<0.002). However, patients with a high tumor burden had similar responses to therapy in terms of disease control rates and progression-free survival.

The authors, therefore, concluded that treatment with 177lutetium-PSMA-617 can be initiated as early as 12 weeks after treatment with radium-223 with an acceptable risk profile and may prolong survival especially in patients with oligometastatic bony disease.
Presented by: Justus Baumgarten, MD, Nuclear Medicine University Hospital Frankfurt Frankfurt Germany


