(UroToday.com) The 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting held in Chicago, IL between June 24th and 27th, 2023 was host to a session on urologic malignancies. Dr. Jeremie Calais presented the results of the ZIRCON phase 3 trial (NCT03849118) that evaluated the test performance characteristics of 89Zr-DFO-girentuximab PET/CT imaging for the accurate detection of clear cell renal cell carcinoma (RCC) primary and secondary lesions in patients with renal masses detected via conventional imaging.
Dr. Calais noted that widespread axial imaging has led to increased incidental detection of renal masses. There is accordingly an unmet clinical need for a non-invasive approach for the diagnosis and characterization of clear cell RCC in such patients. Current anatomic imaging cannot reliably distinguish between benign and malignant renal masses. Twenty to thirty percent of resected small renal masses are ultimately found to be benign, with partial nephrectomies in such patients associated with complication rates as high as 30%. Renal mass biopsies are invasive, associated with potential complications including hematoma and pneumothorax, may theoretically lead to cancer seeding, and may be non-diagnostic in 10-15% of cases (insufficient tissue) and/or associated with a sampling error (negative predictive value of 70%).1 Why focus on clear cell RCC histologic subtype specifically? Clear cell histology accounts for ~75% of all RCC masses and causes ~90% of deaths. Furthermore, among patients with small renal masses on active surveillance, clear cell RCC subtype is associated with the most rapid linear growth rate.2 Accordingly, it becomes clear that identification of a clear cell RCC-specific cell surface marker allowing for targeted imaging techniques in this disease setting is of utmost importance.
Carbonic anhydrase IX (CAIX) is a transmembrane glycoprotein involved in oxygen sensing pathways and has a functional role in tumor acid/base regulation. It is expressed in many hypoxic solid tumors and under hypoxic conditions activates HIF-1 mediated signaling cascades, leading to the activation of hypoxia-regulated genes. This leads to cell metabolism reprogramming (‘glycolic switch’), with the production and export of lactate, which leads to a decline of the extracellular environment pH. The acidic extracellular space promotes tumor cell invasiveness, with hypoxic conditions correlated with disease progression and therapy resistance. CAIX has low expression levels in normal tissues, making it an attractive potential ‘pan-cancer’ target. Significantly, CAIX is expressed 90% of clear cell RCC tumors.
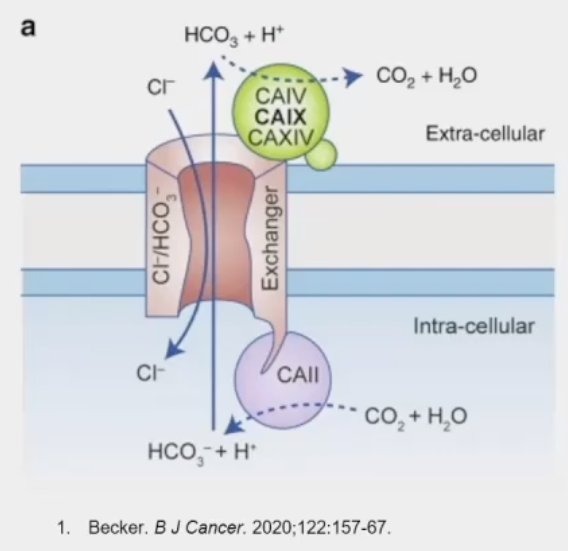
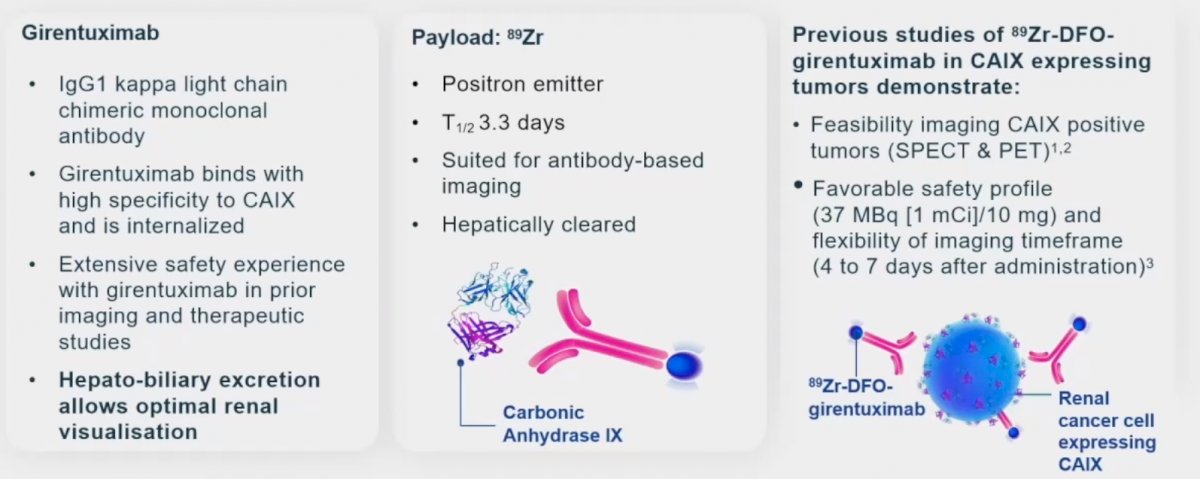
89Zr-DFO-girentuximab (TLX250-CDx) is an antibody-conjugate PET imaging tracer that specifically targets CAIX. Girentuximab is an IgG1 kappa light chain chimeric monoclonal antibody that specifically binds the CAIX and is subsequently internalized. Girentuximab has been demonstrated to be safe in numerous studies. Significantly, it is excreted via the hepato-biliary route which allows for the optimization of renal visualization. 89Zr is the ‘payload’ positron emitter component. It is also hepatically cleared with a half-life of 3.3 days. This conjugate (89Zr + girentuximab) has previously demonstrated feasibility for the imaging of CAIX positive tumors, using both SPECT and PET imaging modalities. It has a favorable safety profile, and given its clearance characteristics, allows for imaging scheduling flexibility (4 to 7 days after administration).
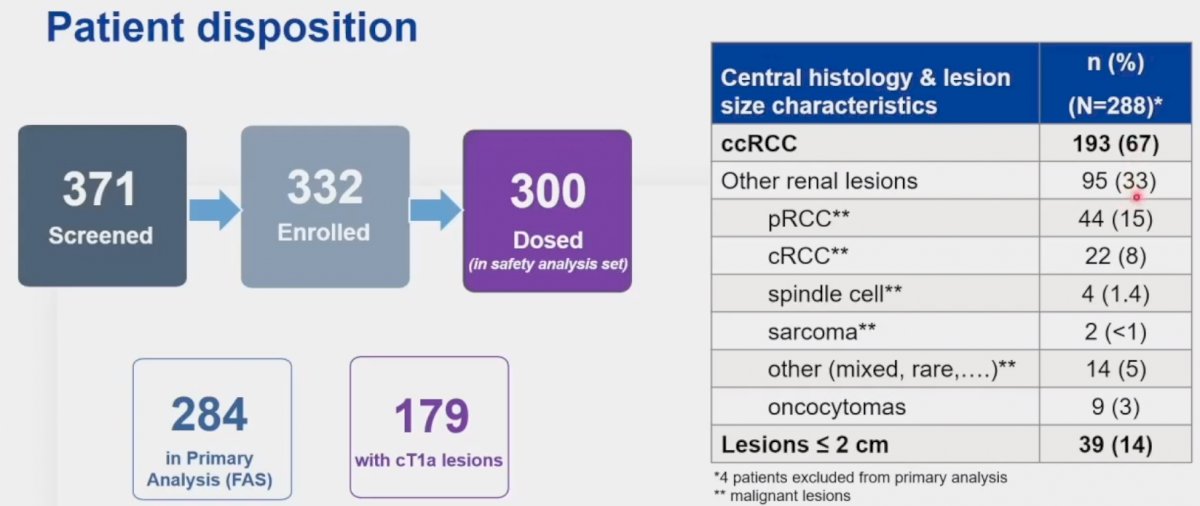
ZIRCON is an international, multicenter trial conducted at 36 sites across nine countries between 2019 and 2022. This trial included patients with a single, indeterminate cT1 renal mass (i.e., ≤7 cm) suspicious for clear cell RCC, detected on CT or MRI. All patients were scheduled for surgical removal (histologic reference standard). Patients received 89Zr-girentuximab on day 0. They subsequently underwent abdominal PET/CT imaging five days (+/- 2) following tracer administration. PET/CT imaging findings were evaluated using a blinded central imaging review. All patients were subsequently planned for partial or radical nephrectomy, with central histology review of surgical specimens, within 90 days of tracer administration.
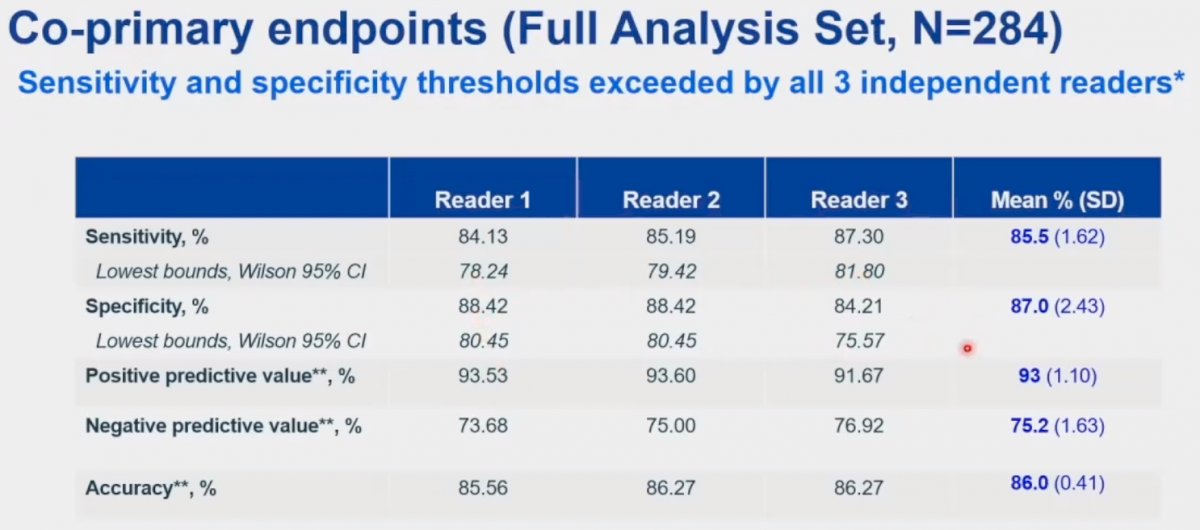
The co-primary endpoints were the sensitivity and specificity of 89Zr-DFO-girentuximab PET/CT for the detection of clear cell RCC, using the surgically resected mass as the reference standard. The key secondary endpoints were sensitivity and specificity in the cT1a (≤4cm) subgroup. All scans were assessed by three independent readers. The study would be deemed ‘positive’ if the lower boundaries of the 95% confidence intervals for both sensitivity and specificity exceeded 70% for two of the three independent readers.

Three hundred patients received the tracer (safety analysis set), of whom 284 were included in the primary analytic set (179 cT1a). Of the 288 evaluable patients (4 subsequently excluded), 67% had clear cell RCC. Other histologic subtypes included papillary RCC (15%), chromophobe RCC (8%), and oncocytomas (3%).
For the co-primary endpoints of sensitivity and specificity in the full analysis set, the corresponding values were 85.5% and 87%, respectively. The positive and negative predictive values were 93% and 75.2%, respectively. The overall accuracy in cT1 masses was 86%. As demonstrated below, we note that the lower boundary of all 95% confidence intervals for each reader exceeded 70%. Thus, this study met its co-primary endpoints.
Among the cT1a subset (n=179), similar performance characteristics were observed as summarized in the table below:
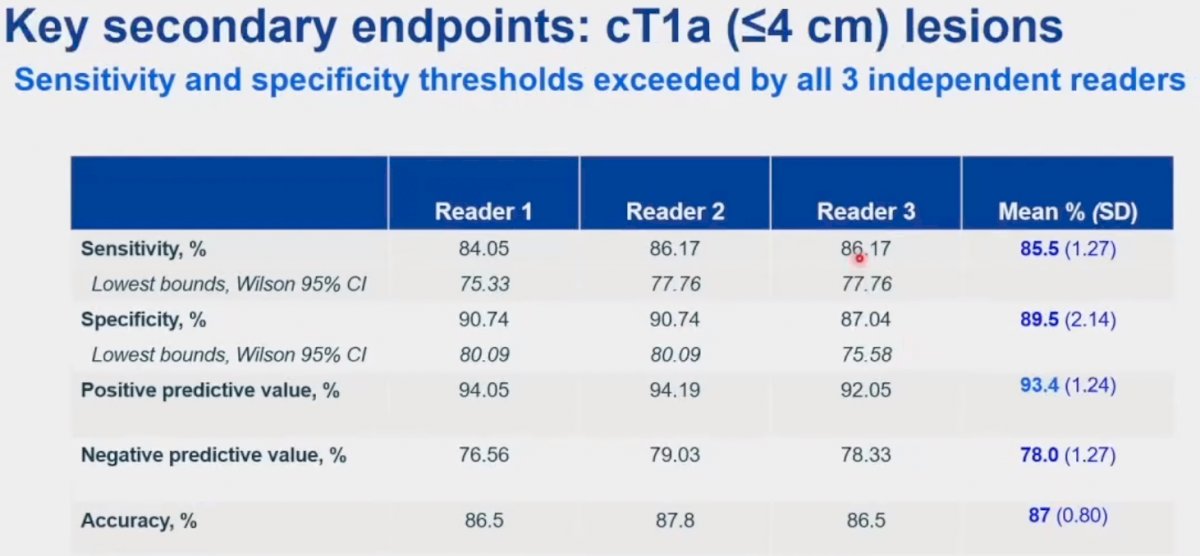
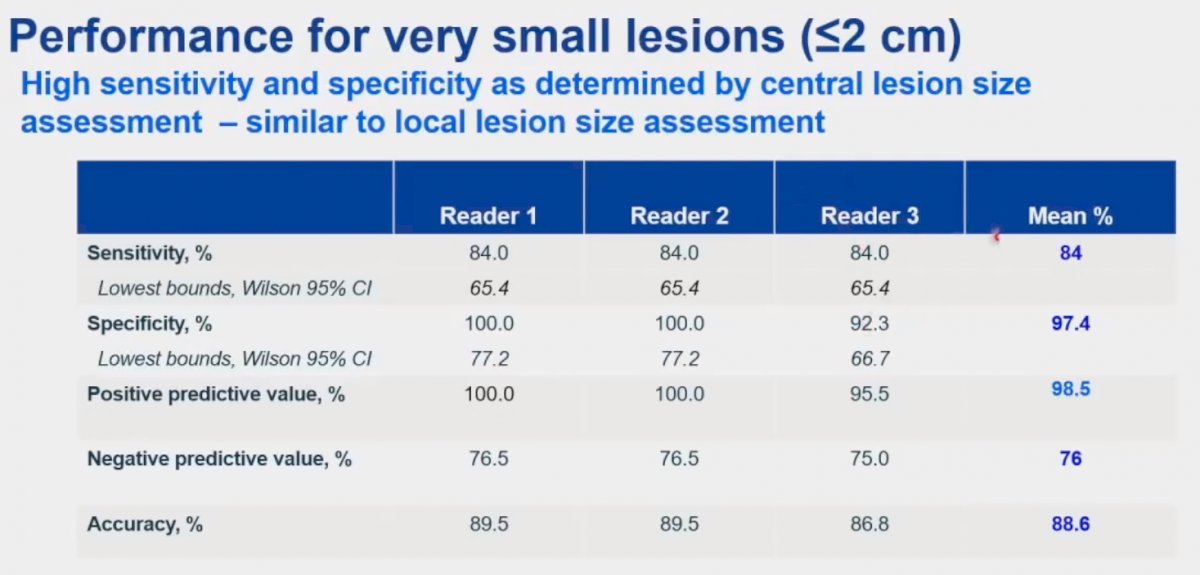
This test demonstrated consistent performance characteristics in the very small lesion cohort (≤2 cm). Given the small subgroup sample size, we note wider 95% confidence intervals with the lower boundary for sensitivity less than 70% for all three readers.
What about inter-reader agreement?
- The inter-reader agreement, quantified using the Fleiss’ Kappa statistic, was 91.1%, which is considerably higher than the commonly accepted cut-off of 70% for ‘acceptable’ agreement. This indicates an almost perfect agreement between the three independent readers when reading the same PET scan (triple-blind reading), suggesting that double/triple blind reading or arbitration will rarely be needed to assess 89Zr-TLX250 PET/CT scans.
- The intra-reader agreement was 100% (Cohen’s kappa statistic), indicating that all three readers were consistent and confident in their findings, and qualitative visual assessment results were completely reproducible.
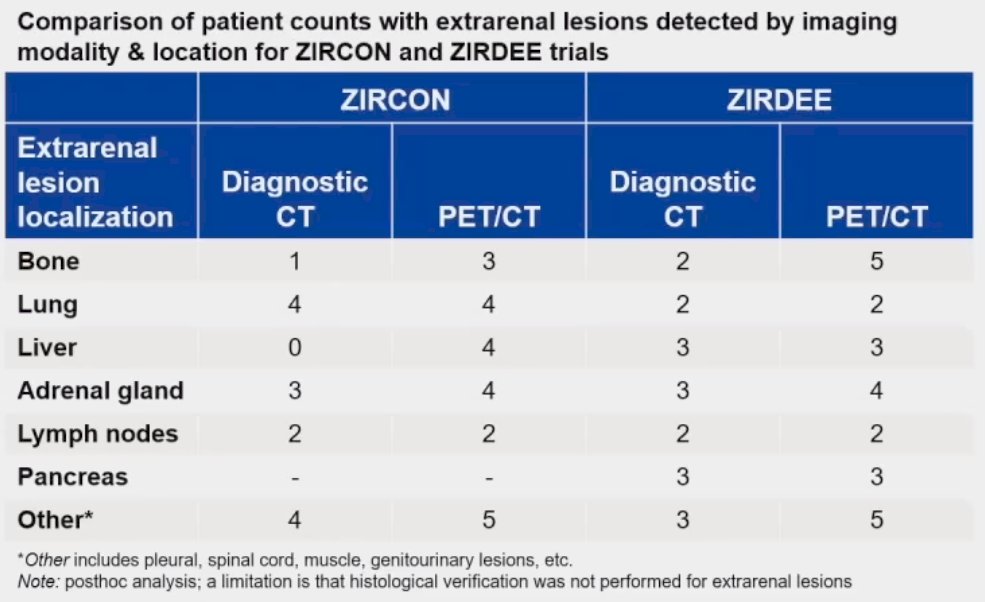
89Zr-DFO-girentuximab PET/CT was also able to detect extrarenal lesions more often than conventional CT (25 versus 14), particularly bone and hepatic metastatic lesions. These findings are comparable to those from the ZIRDEE trial for PET/CT in this setting.3
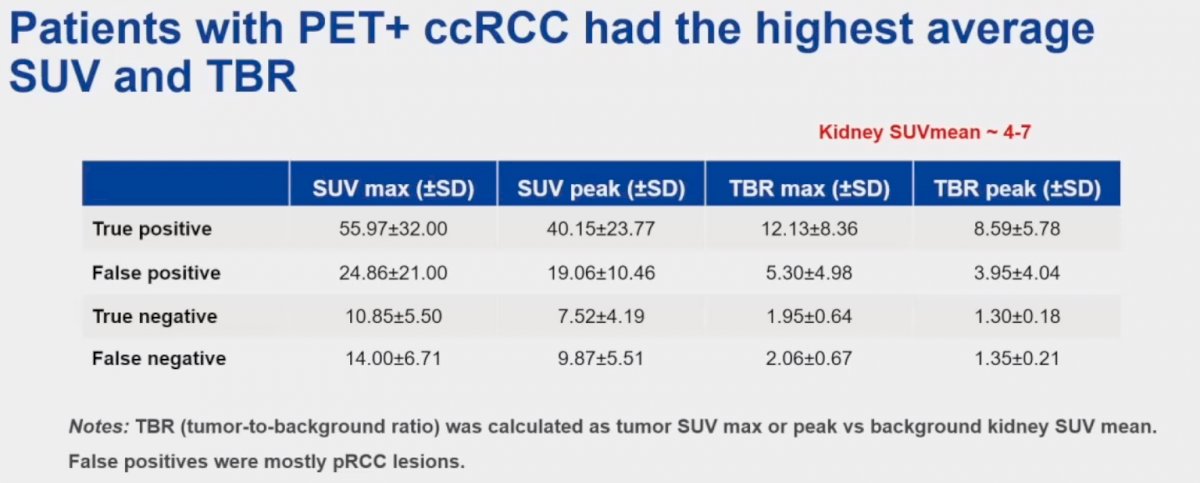
Patients with PET-positive clear cell RCC had the highest average standardized uptake values (SUV) and tumor-to-background ratios (TBR). This is summarized in the table below, whereby the SUVmax, SUVpeak, TBR max and TBR peak values for true positive lesions were 56, 40.2, 12.1, and 8.6, respectively.
The ZIRCON trial confirms the prior known safety and tolerability profile of 89Zr-DFO-girentuximab. There were very few tracer-related adverse events and only 18 (6%) patients had grade 3 or worse treatment-emergent adverse events. The adverse event profile was consistent with post-surgical complications related to the nephrectomy, and no unexpected safety signals were observed.

In summary, the ZIRCON phase 3 trial met its primary endpoints, with sensitivity and specificities ≥84% in all three independent readers (86% an 87% overall, respectively). Significantly, the lower boundaries of the 95% confidence intervals for both sensitivity and specificity among all three readers exceeded 70% (condition for trial ‘positivity’). Furthermore, 89Zr-DFO-girentuximab-labeled PET/CT demonstrated high sensitivity and specificity for the detection and characterization of cT1a (≤4 cm) and ‘small’ (≤2 cm) renal masses. This study confirms the favorable safety and tolerability profile of the 89Zr-DFO-girentuximab tracer. There was evidence of high inter- and intra-reader agreement, and this imaging modality demonstrates potential utility for whole-body staging and disease localization.
Presented by: Jeremie Calais, MD, MSc, Associate Professor of Nuclear Medicine and Theranostics, Department of Molecular and Medical Pharmacology, University of California, Los Angeles, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
References:- Patel et al. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J Urol, 2016;195(5):1340-7.
- Finelli et al. Small Renal Mass Surveillance: Histology-specific Growth Rates in a Biopsy-characterized Cohort. Eur Urol, 2020;78:460-7.
- Hinkman et al. Eur Urol, 2018;257-60.


