(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Raksha Kulkarni discussing 177Lu-PSMA-617 therapy in chemotherapy naïve mCRPC patients. The FDA recently approved 177Lu PSMA 617 therapy for mCRPC patients based on results of the VISION trial, which showed that therapy prolongs overall survival by 15.3 months.1
However, patients with contraindications to chemotherapy from coexisting morbidities are ineligible to receive this therapy and thus have restricted therapeutic options. As our understanding of factors contributing to therapy response and patient outcomes improves, patient selection for 177Lu PSMA 617 radioligand therapy should also evolve to include more patients who would benefit from this therapy. At the SNMMI 2023 annual meeting, Dr. Kulkarni and colleagues reported results of their study of chemotherapy naïve mCRPC patients who received radioligand therapy at their institute.
Mount Sinai Medical Center participated in the Managed Access Program (MAP) (NCT04825652) to provide radioligand therapy to mCRPC patients prior to its FDA approval (September 2021 to March 2022). Patients who had not received prior taxane-based chemotherapy were excluded from the MAP, and Dr. Kulkarni and colleagues obtained a single-patient investigational new drug approval from Novartis Pharmaceuticals® and the Food and Drug Administration for radioligand therapy for patients with contraindications to chemotherapy. Characteristics evaluated included pre-therapy disease characteristics (Gleason score and PSA levels at diagnosis), 18F-DCFYPyL PET CT imaging characteristics (total tumor volume, total lesion activity, and SUVmean), response to 177Lu PSMA 617 (decrease in PSA at 4 weeks after the second dose) and hematologic toxicity (pre-therapy and worse hematological toxicity grade assessed at 2-4 weeks after 2 cycles of therapy). Hematological toxicity was graded according to the National cancer institute's common terminology criteria for adverse events.
There were 17 patients who received radioligand therapy after at least one cycle of taxane-based chemotherapy (MAP) and 9 patients who were chemotherapy naïve and received the radioligand therapy under single patient investigational new drug approval. The baseline characteristics of these patients are as follows: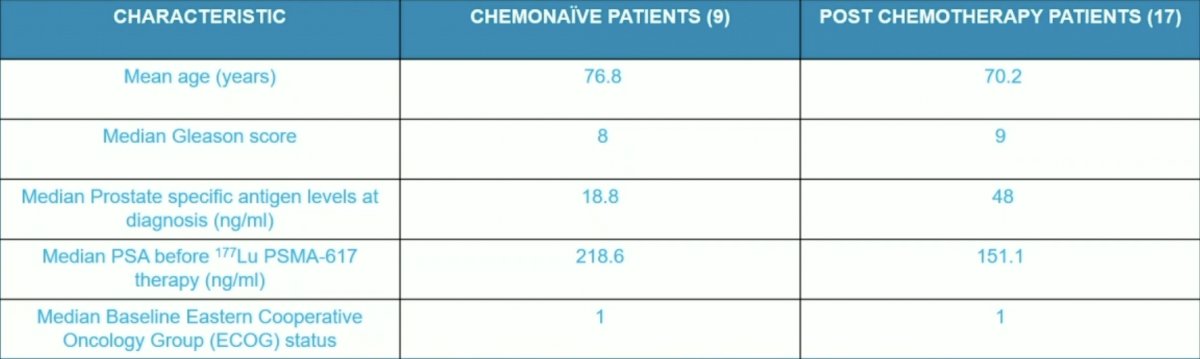
Additionally, prior treatments in the chemotherapy and post-chemotherapy patients is as follows: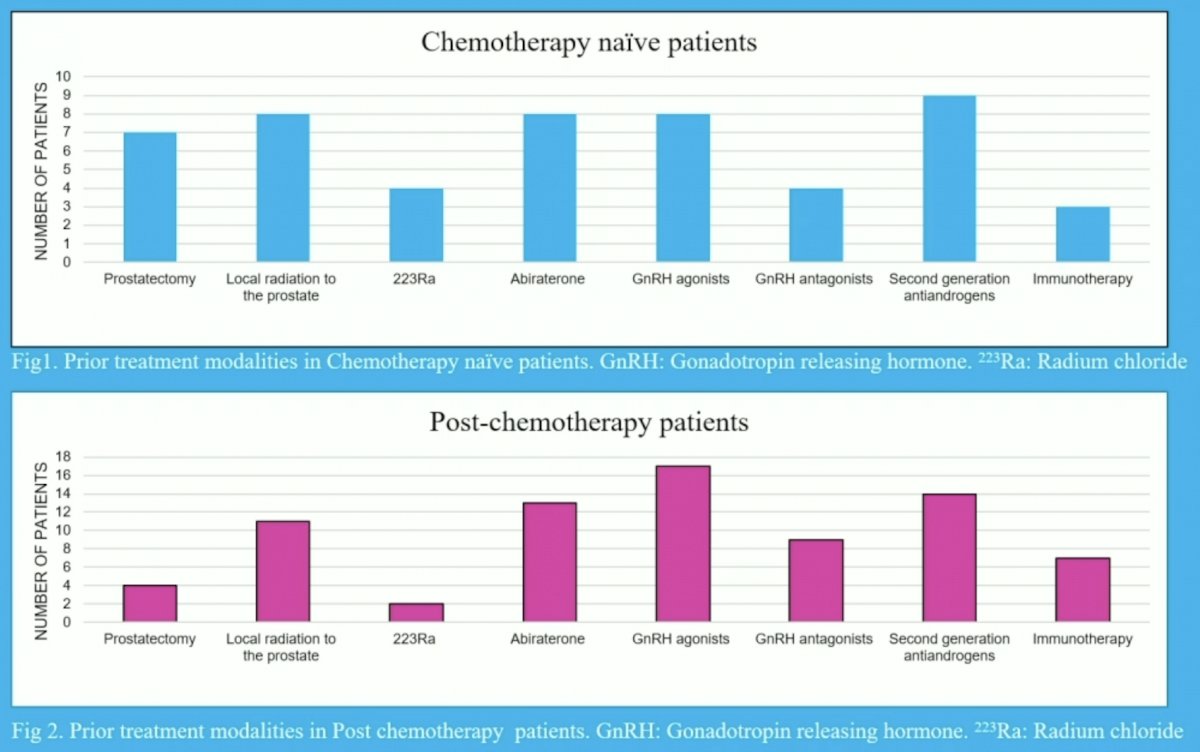
Pre-therapy 18F-DCFYPyL PET CT imaging characteristics are as follows for these patients: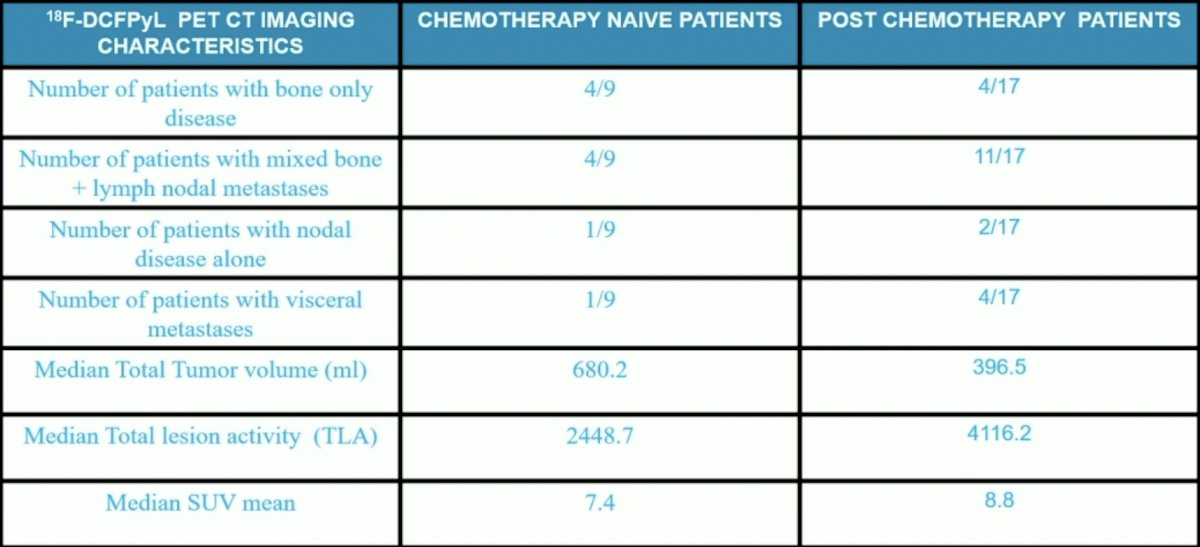
8/17 MAP and 3/6 chemotherapy naïve patients completed 6 cycles of therapy:
- 47% of MAP and 22.2% of chemotherapy naïve patients stopped treatment due to progression of disease
- 11.7% MAP patients and 22.2% chemotherapy naïve patients stopped treatment due to toxicity
- 11.7 % MAP and 22.2% chemotherapy naïve patients achieved complete response
- The remaining stopped voluntarily or due to blood dyscrasias unrelated to toxicity
There were 77.8% (7/9) of chemotherapy naïve patients and 52.9% (9/17) of MAP patients that had a reduction in PSA after 2 cycles. Of the patients who had a decrease in PSA, 28.5% (2/7) of chemotherapy naïve patients and 88.8% (8/9) MAP patients had a >50% reduction. No chemotherapy naïve patients had thrombocytopenia of > grade 4, while 5.8% of MAP patients had thrombocytopenia of > grade 4. Furthermore, 11.7% of MAP patients had anemia of > grade 4, whereas 11.1 % chemotherapy naïve patients developed anemia > grade 4. None of the chemotherapy naïve patients but 17.6% of MAP patients had leukopenia of > grade 3. Ultimately, there was no statistically significant difference between degree of thrombocytopenia (p = 0.26), leukopenia (p = 0.90), or anemia between the two subsets, (p = 0.12):
Several limitations of this study included the small sample size and no long term follow up to date for these patients.
Dr. Kulkarni concluded her presentation discussing 177Lu-PSMA-617 therapy in chemotherapy naïve mCRCP patients by emphasizing that in this heterogeneous population of heavily pretreated patients, a small group of chemotherapy naïve patients had a similar toxicity profile and response to 177Lu-PSMA-617 therapy as compared to the post-chemotherapy subset.
Presented by: Raksha Kulkarni, MD, Icahn School of Medicine, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
References:


