(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Andrew Nguyen discussing Re-SPECT, a study assessing patient outcomes following a response biomarker guided approach to treatment using 177Lu-PSMA-I&T in men with metastatic castrate resistant prostate cancer (mCRPC). 177LuPSMA is an effective treatment in mCRPC with trials, such as VISION and TheraP, adopting a standardized dose interval.1,2
However, adjusting treatment intervals utilizing early response biomarkers may improve patient outcomes. Early PSA response has been associated with better overall survival, and patients with a >=20% increase in volume + the appearance of new lesions at 12 weeks (RECIP criteria) have worse survival (8.3 months vs 13 months). As such, this study evaluated progression-free survival and overall survival based on treatment interval adjustment utilizing 177LuPSMA 24-hour SPECT/CT (177Lu-SPECT) and early PSA response.
This was a retrospective study (May 2019 to April 2022) of 125 men treated on a clinical program with 6-weekly 177LuPSMA-I&T (median 3 cycles, IQR 2-4) doses, with a median dose 8.0 GBq (95%CI 7.5-8.0). Inclusion criteria included progression on at least one line of androgen receptor signaling inhibition, either failed or were eligible for taxane chemotherapy, ECOG 0-2, eGFR >30 ml/min, hemoglobin > 70 g/L, and platelets > 70,000. Imaging screening involved 68GaPSMA-11 PET/diagnostic CT, requiring the following:
- SUVmax >= 15 on PSMA PET at >= 1 site
- SUVmax >=10 at all measurable sites
- No sites of PSMA negative soft tissue metastatic disease on diagnostic CT
177Lu-SPECT/diagnostic CT were acquired following each therapy, and clinical assessments three times weekly. Following dose 2 (week-6), a composite of PSA and 177Lu SPECT/CT response (partial response, stable disease and progressive disease) determined ongoing management:
- Response group 1 (marked reduction in in PSA/imaging partial response): cease treatment until subsequent PSA rise, then re-treatment
- Response group 2 (stable or reduced PSA and/or imaging stable disease): 6 weekly treatment until 6 doses or no longer clinically benefitting
- Response group 3 (rise in PSA and/or imaging progressive disease): recommend change to an alternative treatment
Imaging response criteria were as follows:

Among the 125 men included in the study, the median age was 75 years with the full baseline characteristics as follows:

Overall PSA50% response rate was 60% (75/125), median PSA-progression-free survival was 6.1 months (95% CI 5.5-6.7) and median overall survival was 16.8 months (95% CI 13.5-20.1). Stratified by response group, 35% (41/116) were classified as response group 1, 34% (39/116) response group 2, and 31% (36/115) response group 3. PSA50% response rate by response group were 93% (38/41) for response group 1, 74% (29/39) for response group 2, and 8% (3/36) for response group 3. The median PSA-progression-free survival for response group 1 was 12.1 months (95% CI 9.3-17.4), 6.1 months (95% CI 5.8-9.0) for response group 2, and 2.6 months (95% CI 1.6-3.1) for response group 3:

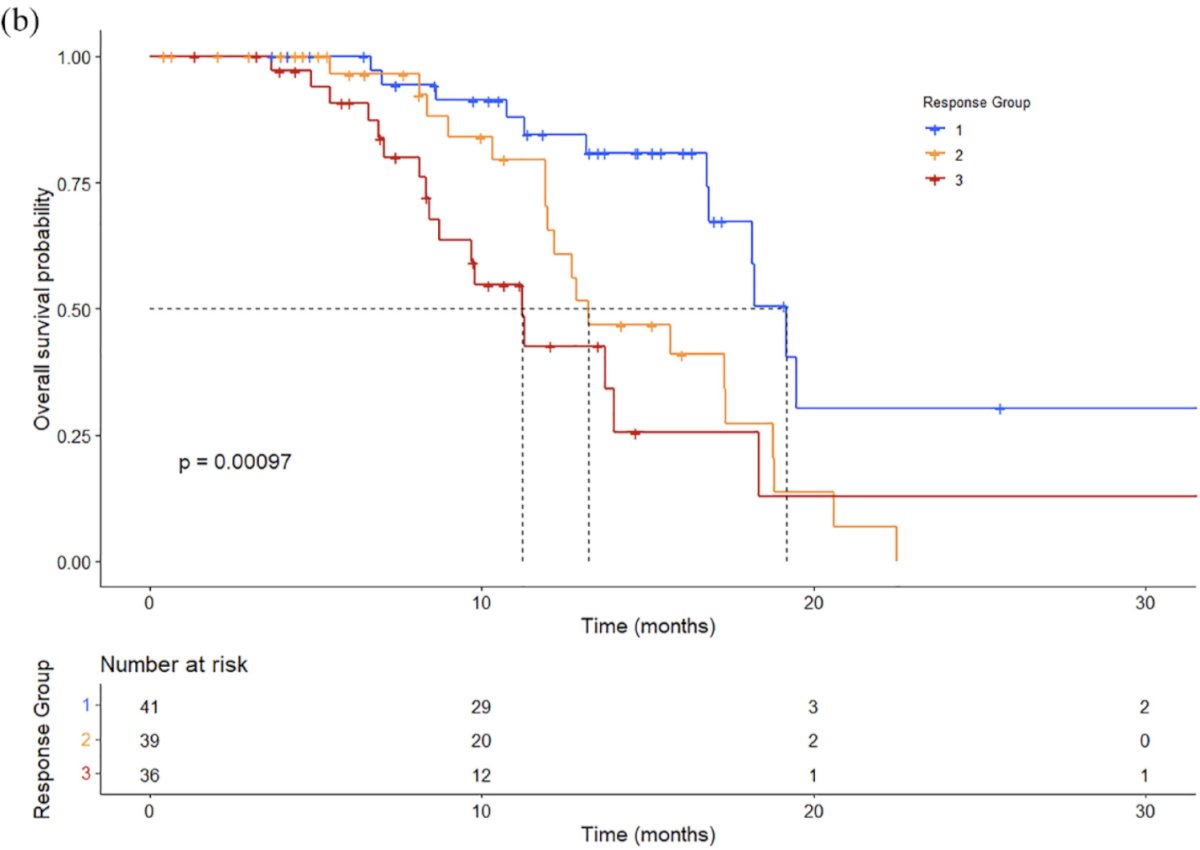
Overall survival was 19.2 months (95% CI 16.8-20.7) for response group 1, 13.2 months (95% CI 12-18.8) for response group 2, and 11.2 months (95% CI 8.7-15.6) for response group 3:
The median months of 'treatment holiday' for response group 1 was 6.1 months (IQR 3.4-8.7). Additionally, 9 men received prior 177Lu PSMA-617 and were retreated with 177Lu PSMA I&T with a PSA50% response rate of 56% on re-treatment. There were several limitations of this study, including the retrospective design, some patients in response group 1 after a treatment holiday electing not to have more treatments, and the definition of visual disease response on SPECT not being well defined in >30% of patients.
Dr. Nguyen concluded his presentation discussing Re-SPECT with the following take home messages:
- Personalizing dosing intervals using early response biomarkers with 177LuPSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early cross-over to potentially more effective therapies in non-responders
- Further evaluation in prospective trials is warranted
Presented by: Andrew Nguyen, St. Vincent's Hospital, Sydney, Australia
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.


