(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Anushna Babu discussing dual PSMA/FDG PET/CT for assessing eligibility and predicting response in mCRPC patients undergoing 177Lu-PSMA-617 radioligand therapy. Even though baseline PSMA expression is a requisite to successful radioligand therapy in metastatic castration-resistant prostate cancer (mCRPC), there is a subset of patients who do not respond to therapy.
In this study presented at SNMMI 2023, Dr. Babu and colleagues sought to compare the eligibility status of mCRPC patients for 177Lu-PSMA-617 radioligand therapy using dual 68Ga-PSMA-11 and 18F-fluorodeoxyglucose (18F-FDG) PET-CT with that obtained through the VISION trial criteria.1 Additionally, they aimed to evaluate PSMA/FDG tumor heterogeneity as a predictor of 177Lu-PSMA-617 radioligand therapy efficacy outcomes.
There were 25 mCRPC patients in this study referred for 177Lu-PSMA-617 radioligand therapy that underwent both 68Ga-PSMA-11 and 18F-FDG PET-CT within 2-4 weeks. Eligibility status for PSMA-radioligand therapy was determined based on the VISION trial criteria (PSMA-PET-based) as well as the interpretation of dual tracer PET-CT (ie. patients with any discordant PSMA-negative/FDG-positive lesion that were deemed ineligible for radioligand therapy). For the eligible patients who underwent PSMA-radioligand therapy, both PET-CT scans were assessed for visual and quantitative heterogeneity parameters. Images were visually evaluated using four categories:
- FDG positive/PSMA negative: One or more discordant lesion which was FDG avid and PSMA non avid
- PSMA ≤ FDG: One or more metastatic lesion showing PSMA uptake less than or equal to FDG
- PSMA > FDG: metastatic lesions which were more PSMA avid than FDG avid
- PSMA positive/FDG negative: purely PSMA avid disease without FDG uptake in any lesion
Quantitative analysis was done with disease extent score and Standardized Uptake Value (SUVmax) of the hottest lesion. The distribution of each tracer was recorded in eight skeletal segments, one visceral segment, and one lymph nodal segment. Furthermore, the extent of disease involvement in each segment was scored on a 0–5 scale. The difference in PSMA disease extent and FDG disease extent score (PSMA-FDG disease extent score) was calculated for each patient. Median values of PSMA-FDG disease extent score, PSMA SUVmax and FDG SUVmax were taken as arbitrary cut-offs to further categorize patients. The primary treatment end-point was progression-free survival, which was calculated using Kaplan-Meier survival analysis and compared across groups using Tarone-Ware test.
Based on both the VISION and the dual tracer criteria, 21/25 (84%) patients were deemed eligible for PSMA-radioligand therapy while 4/25 (16%) patients were considered ineligible (kappa = 1.0, perfect agreement). Of the 17 patients who underwent radioligand therapy, nine had PSMA > FDG and eight had PSMA ≤ FDG disease. None of the patients who underwent radioligand therapy had purely FDG positive/PSMA negative or PSMA positive/FDG negative disease. The median progression-free survival for patients with PSMA > FDG disease was 9.0 months (95% CI 2.8-15.3) compared to 4.0 months (95% CI 3.4-4.6) for patients with PSMA ≤ FDG disease (p = 0.034):
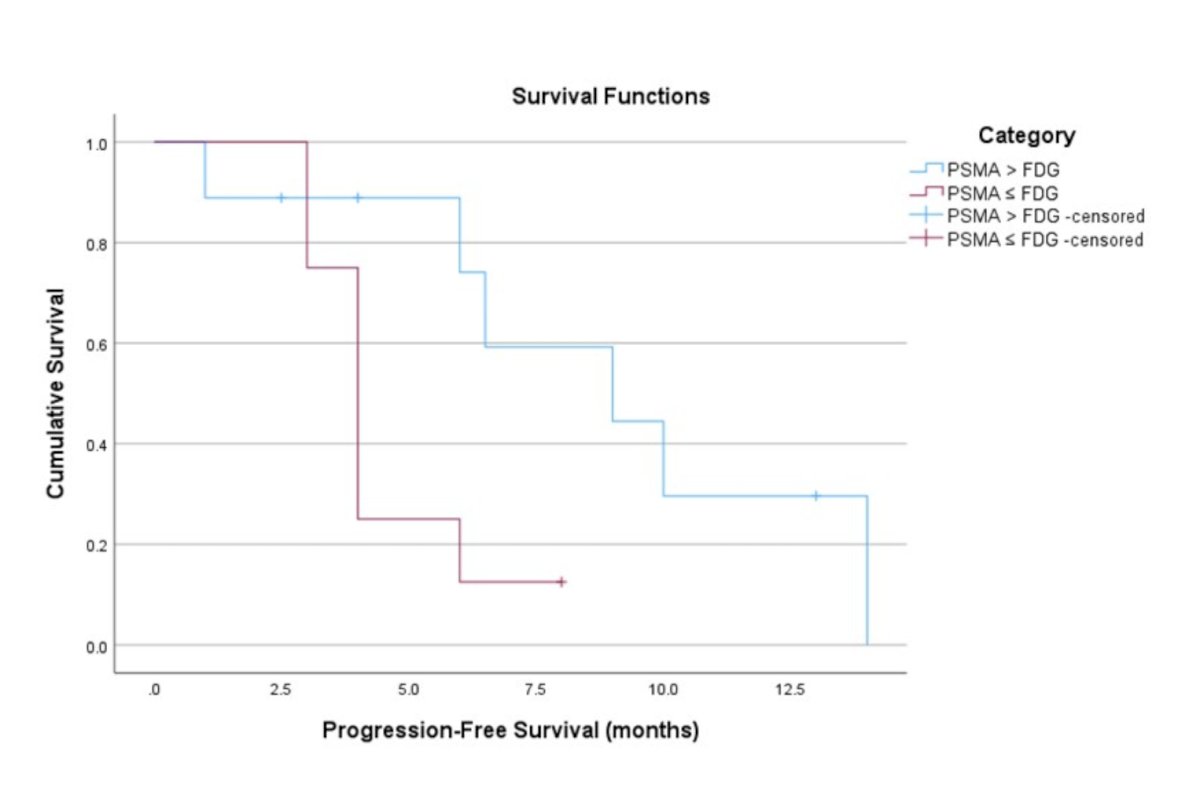
However, none of the quantitative heterogeneity parameters were significantly associated with progression-free survival. Patients with difference in PSMA-FDG disease extent score <6 had median progression-free survival of 4.0 months (95% CI 1.9-6.1) while patients with PSMA-FDG disease extent score ≥6 had median progression-free survival of 6.5 months (95% CI 2.7–10.3) (p = 0.594). Median progression-free survival of patients with PSMA SUVmax <26.8 was 4.0 months (95% CI 3.1–4.8) versus 6.5 months (95% CI 4.0–8.9) for patients with PSMA SUVmax ≥ 26.8 (p = 0.117). Similarly, the median progression-free survival of patients with FDG SUVmax <11.4 was 9.0 months (95% CI 2.6–15.4) versus 4.0 months (95% CI 3.0–4.9) for patients with FDG SUVmax ≥11.4 (p = 0.115). A summary of the visual and quantitative heterogeneity parameters and progression free survival outcomes is as follows:
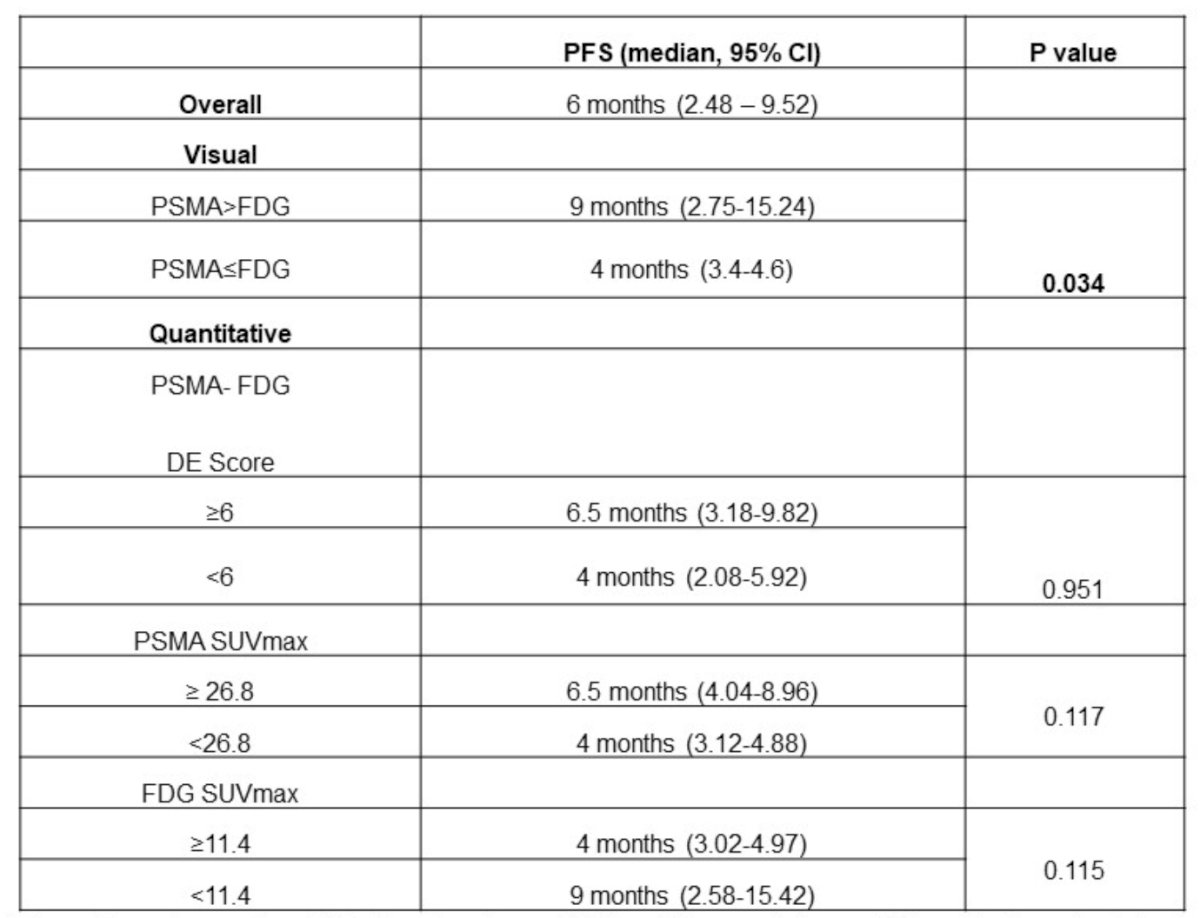
Dr. Babu concluded this presentation discussing dual PSMA/FDG PET/CT for assessing eligibility and predicting response in mCRPC patients undergoing 177Lu-PSMA-617 radioligand therapy with the following take home messages:
- Dual tracer PSMA/FDG PET-CT did not provide additional information over PSMA-only analysis for assessing patient suitability for PSMA-radioligand therapy
- Visually assessed tumor heterogeneity on dual tracer PET-CT seems to be a significant predictor of outcomes post-radioligand therapy
Presented by: Anushna S. Babu, MD, AIIMS, New Dehli, India
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
References:


