(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Isabel Rauscher discussing the extension of a 68Ga-PSMA PET-based nomogram for outcome prediction of 177Lu-PSMA radioligand therapy for the use of 18F-rhPSMA -7.3.
Recently, 177Lu-PSMA radioligand therapy has been approved by the FDA and EMA for patients with metastatic-castration resistant prostate cancer (mCRPC) based on the phase 3 VISION trial.1 Given that a response to 177Lu-PSMA radioligand therapy is not achieved by all patients, nomograms, including pre-therapeutic 68Ga-PSMA-PET, were recently established to predict outcomes:2
However, the increasing use of 18F-labelled PSMA-ligands requires adaptation of these methods. The aim of this retrospective analysis was to assess application of the 68Ga-PSMA-PET-based recently published nomogram for use with 18F-rhPSMA- 7.3 (POSLUMA®) prior to Lu-PSMA radioligand therapy in a large cohort of patients with mCRPC.
A total number of 174 patients (median age 74, interquartile range 68-80 years) were retrospectively included in this study. Parameters included the same as the 68Ga-PSMA PET nomogram clinical variables (time since diagnosis, chemotherapy status, baseline hemoglobin) and imaging data now derived from 18F-rhPSMA-7.3 PET (tumor SUVmean, >=20 metastatic lesions, presence of pelvic lymph node, bone and/or liver metastases). Pre-therapeutic outcome probabilities (PSA-progression-free survival and overall survival) for each patient were estimated according to the published 68Ga-PSMA PET-based prediction models [2]. To assess the use of 18F-rhPSMA-7.3 in this nomogram, the observed and estimated outcome of each patient c-index were calculated for overall survival and progression-free survival. Finally, overall survival and progression-free survival were compared, stratifying patients in high- and low-risk groups based on the cut-off values determined by Gafita et al [2].
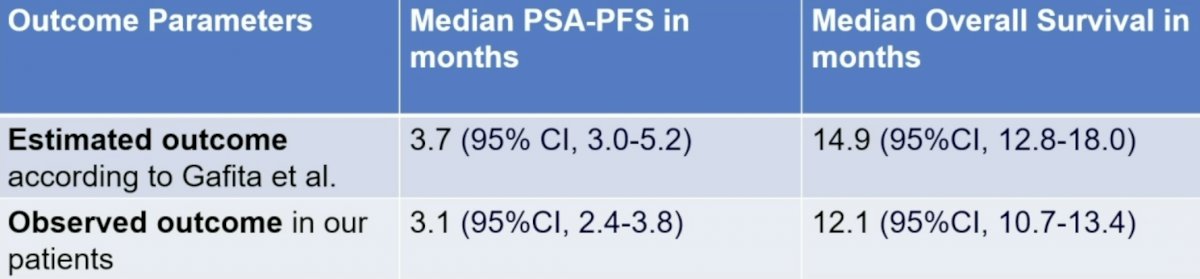
The estimated median progression-free survival and overall survival were 3.7 months (95% CI, 3.0-5.2) and 14.9 months (95%CI, 12.8-18.0), respectively. The observed progression-free survival and overall survival were 3.1 months (95%CI, 2.4-3.8) and 12.1 months (95%CI, 10.7-13.4), respectively:
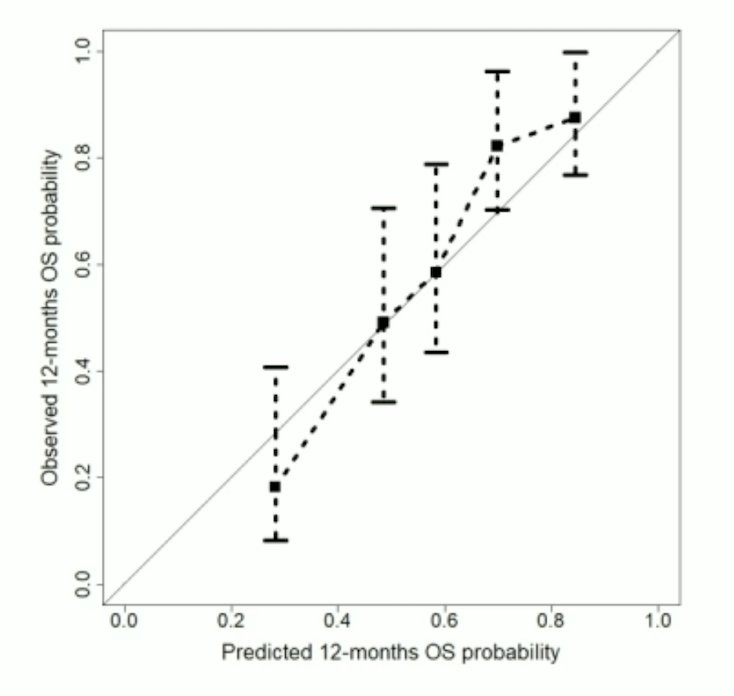
For the overall survival model, this data reached a C-index of 0.70 (95% CI 0.66-0.75), which is comparable to the validation cohort of Gafita et al. with a C-index of 0.72 (95%CI 0.68-0.76):1
The C-index for PSA-progression-free survival was 0.64 (95% CI 0.60-0.70) and substantially lower compared to a C-index of 0.71 (95% CI 0.65-0.78) in the validation cohort of Gafita et al:

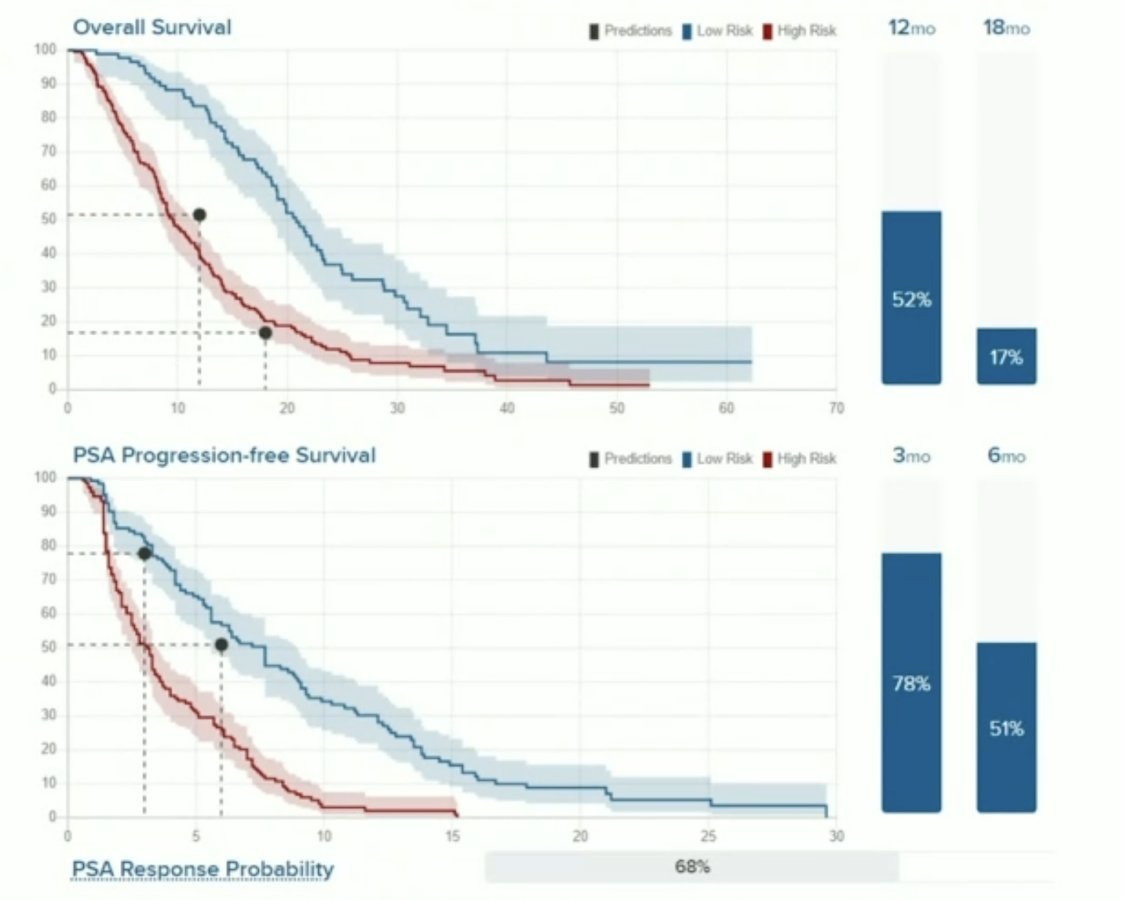
Grouping patients in high- and low-risk showed a strong stratification in short and long term outcomes. Low-risk patients presented with a significantly longer overall survival than high-risk patients (16.5 months (95% CI 15.1-17.8) vs. 8.8 months (95% CI 7.7-9.9), respectively; p < 0.0001). Corresponding PSA-progression-free survival was also significantly longer in the high-risk vs. the low-risk group (5.4 months (95% CI 4.7-6.1) vs. 2.6 months (95% CI 1.9-3.2); p = 0.003).
Dr. Rauscher concluded her presentation discussing the extension of a 68Ga-PSMA PET-based nomogram for outcome prediction of 177Lu-PSMA radioligand therapy for the use of 18F-rhPSMA -7.3 with the following take home messages:
- The use of data from 18F-rhPSMA 7.3-PET substituting 68Ga-PSMA in a recently proposed PET-based prediction nomogram performs well for assessing outcome of 177Lu-PSMA radioligand therapy outcome assessment with similar c-indices for overall survival in comparison to the literature
- However, irrespective of the PSMA-ligand used, the predictive accuracy of the nomogram is suboptimal and should be used with caution the decision on treatment initiation should be based on different factors
- Further improvement of the model is warranted as prediction models might be beneficial in patient selection
Presented by: Isabel Rauscher, MD, Technical University of Munich, Munich, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Gafita A, Calais J, Grogan TR, et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: An international, multicentre, retrospective study. Lancet Oncol. 2021 Aug;22(8):1115-1125.


