(UroToday.com) The 2023 SNMMI annual meeting included a prostate cancer session, featuring a presentation by Dr. Minseok Suh discussing preliminary results of safety, dosimetry, and efficacy in a phase I/II clinical trial of 177Lu-DGUL in metastatic castration-resistant prostate cancer (mCRPC) patients.
PSMA is a transmembrane protein that is overexpressed in prostate cancer cells more than 100-fold to 1,000-fold higher than normal epithelial cells, which makes it an attractive target for theranostics. 177Lu-DGUL is a novel PSMA-targeting tracer based on Glu-Urea-Lys derivatives conjugated to a DOTA chelator:
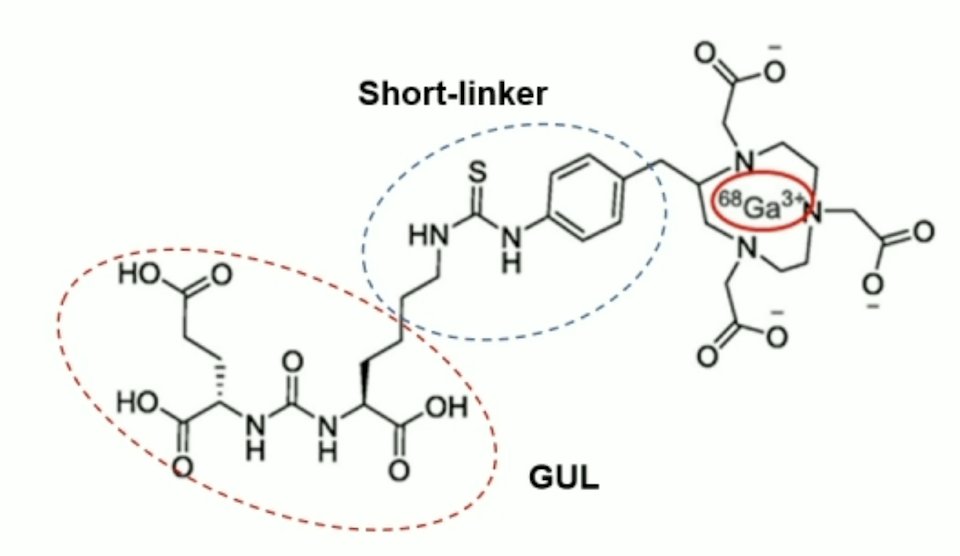
These characteristics result in lower uptake in normal organs, rapid clearance, but still with excellent lesion detection ability:
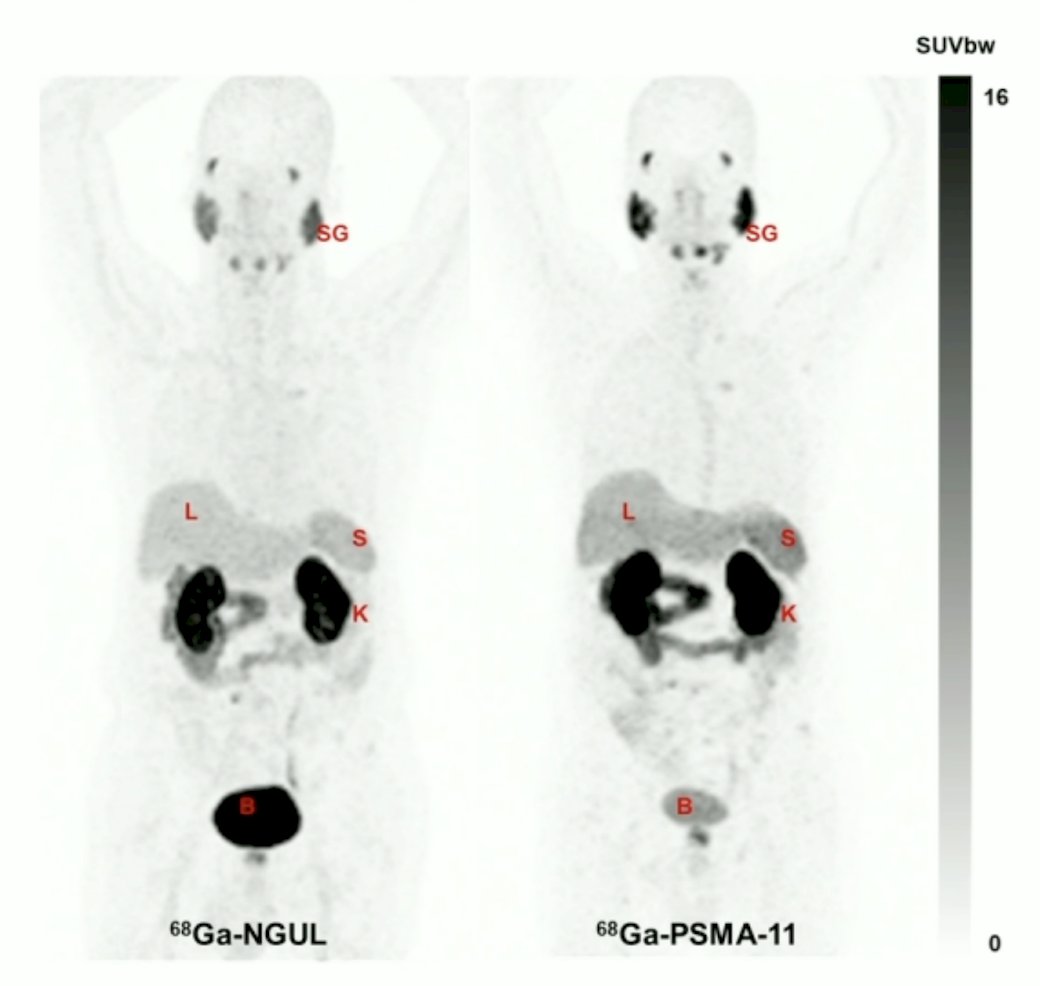
The purpose of this phase I/II clinical trial was to evaluate the safety, tolerability, dosimetry, and anti-tumor activity of Ga-68-NGUL/177Lu-DGUL in patients with mCRPC refractory to standard therapy. At the SNMMI 2023 annual meeting, Dr. Suh and colleagues presented preliminary results from this trial.
The study design was a phase I part A, phase I part B, and a phase 2 trial, evaluating safety, dose-limiting toxicities, and maximum tolerated dose of 177Lu-DGUL in 3+3 dose escalation cohorts with an initial dose of 5.55 GBq and 7.40 GBq. Cohort expansion will enroll at the maximum tolerated dose to assess safety and efficacy:

Eligibility criteria include mCRPC patients who have previously been treated with at least one androgen-receptor–pathway inhibitor and docetaxel and who had a PSMA-positive PET scan. Patients are administered up to 6 cycles of 177Lu-DGUL every 6 weeks. The full treatment protocol is as follows:
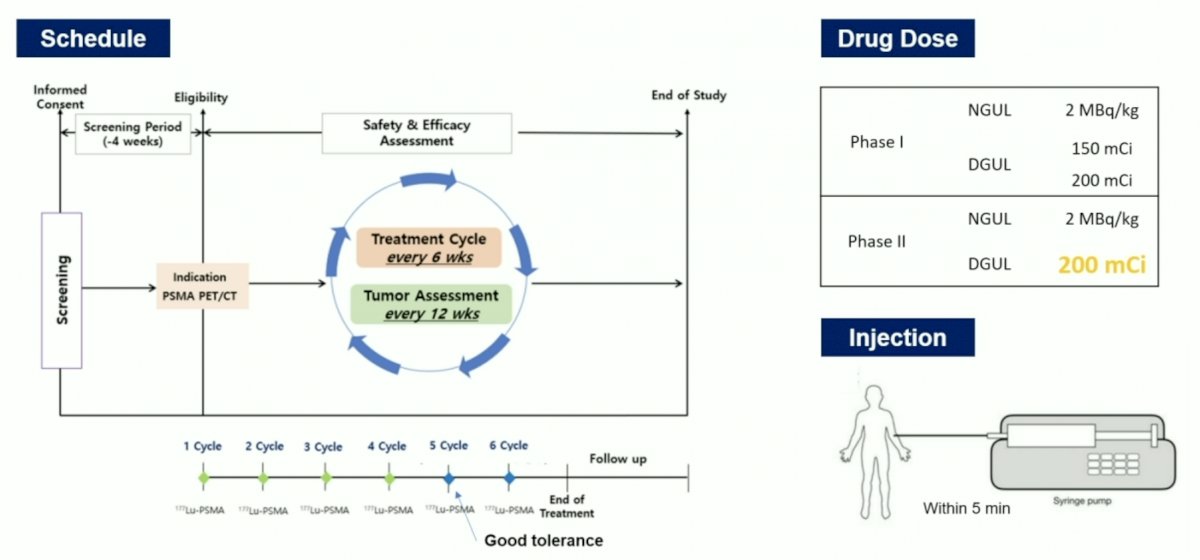
To date, 31 patients have been assessed for eligibility, 25 patients have been enrolled, and 24 patients have received intervention. The baseline patient characteristics are as follows:

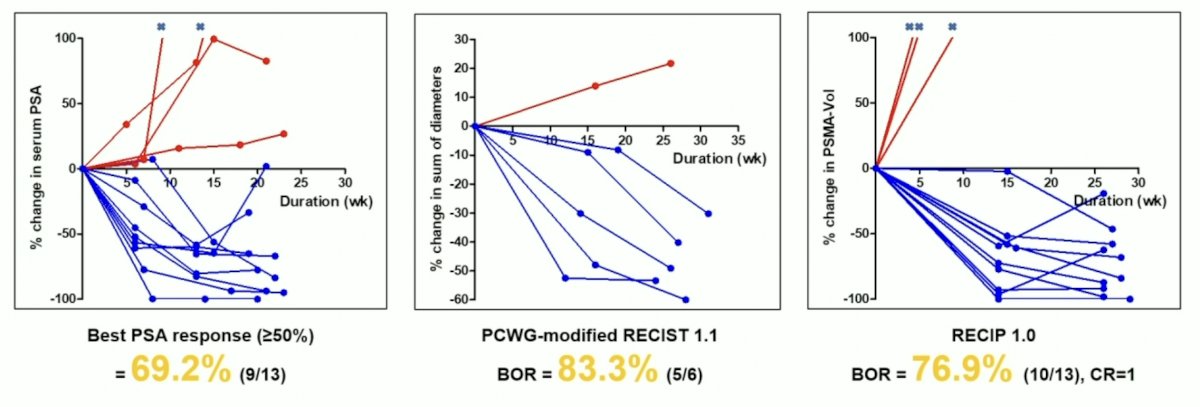
In the phase I dose escalation study, no dose-limiting toxicities was observed and the maximum tolerated dose was 7.40 GBq. The mean absorbed doses to salivary glands, kidneys, and red marrow were 0.32 Gy/GBq, 0.31 Gy/GBq, and 0.02 Gy/GBq, respectively. With follow-up ongoing, a PSA decline of more than 50% was observed in 52.2% (12/23) of patients:

Among patients receiving at least 2 cycles of treatment, and with image based response evaluation (13 with evaluable disease, 6 with measurable disease), a PSA decline of more than 50% was seen in 69.2% (9/13) of patients, with excellent (76.9%-83.3%) objective response:
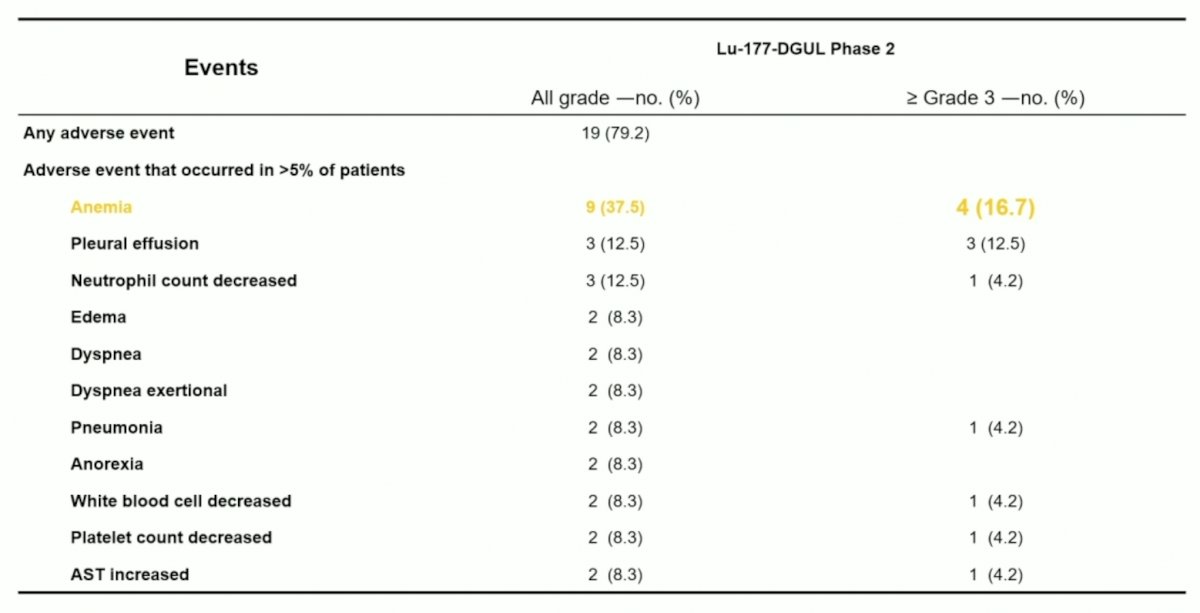
Until now, no severe adverse drug reactions have been observed. Overall 79.2% of patients had an all-grade adverse event, most commonly anemia (37.5% all grade; 16.7% grade >= 3) and pleural effusion (12.5% all grade; 12.5% grade >= 3):
Dr. Suh concluded this presentation discussing preliminary results of safety, dosimetry, and efficacy in a phase I/II clinical trial of 177Lu-DGUL in mCRPC patients with the following take home messages:
- Treatment with 177Lu-DGUL was well tolerated, with no treatment related deaths and few treatment related adverse events of grade 3 or higher (anemia was the most frequent)
- Mean absorbed doses to the salivary glands, kidneys and the red marrow were 0.32 Gy/Gbq, 0.31 Gy/Gbq, and 0.02 Gy/GBq, respectively
- The best PSA response was achieved in 52.2% of patients (12/23) and objective response was achieved in 83.3% of patients (5/6) with measurable disease
- Recruitment for a phase 2 expansion cohort is underway to further evaluate the safety and objective response rate according to RECIST v1.1
Presented by: Min Seok Suh, MD, PhD, FANMB, Seoul National University Hospital, Seoul, South Korea
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.


