(UroToday.com) The 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting held in Chicago, IL between June 24th and 27th, 2023 was host to a prostate cancer session. Dr. Ahmad Abdelrazek presented the results of a real-world, retrospective analysis evaluating the early adverse events, emergency room visits, and hospitalizations following prostate-specific membrane antigen (PSMA)-based radioligand therapy (RLT).
Lutetium-177-PSMA (Lu-PSMA) is an established, effective and safe treatment option for the management of metastatic castrate-resistant prostate cancer (mCRPC) patients who have progressed following prior taxane-based chemotherapy and androgen receptor signaling inhibitors.1,2 The majority of available data regarding adverse effects/safety profile originate from clinical trial settings, and, as such, real world data is lacking. The objective of this study was to report all adverse events occurring during or shortly after Lu-PSMA treatment and to explore potential variables independently associated with emergency room visits and hospital admissions.
The investigators retrospectively reviewed the medical records of 185 patients at the Mayo Clinic in Rochester, MN, receiving Lu-PSMA between April and November 2022. The primary objective was to evaluate/report the early treatment side effects, as outlined in the clinical notes. The secondary objective was to evaluate emergency room visits and hospitalizations within 30 days of Lu-PSMA administration. Univariable and multivariable logistic regression analyses were used to evaluated independent variables associated with emergency room visits and hospital admissions.

The median patient age at treatment initiation was 70 years (range: 67 to 76) and the median PSA level was 11.8 ng/ml (range: 2.3 to 76.4 ng/ml). The majority of patients had ECOG performance status of 0 or 1 (92.5%). Lymph node, bone, and visceral metastases, as per 68Ga or 18F-DCFPyL-PSMA PET/CT, were present in 67%, 89%, and 32% of patients.
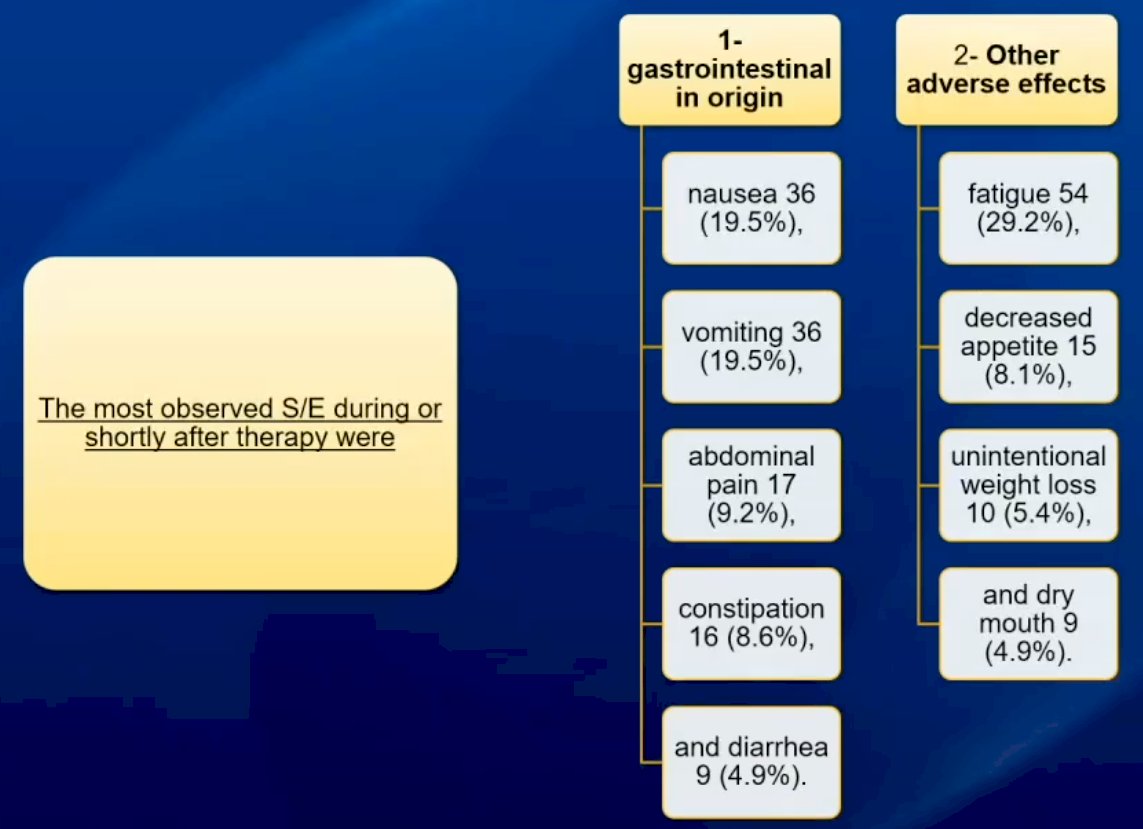
The most commonly observed adverse events were gastrointestinal in nature (nausea, vomiting, abdominal pain, etc.), as summarized in the flow chart below.
Emergency room visits were recorded for 16 patients (8.6%), with the following documented reasons:
- Anemia and thrombocytopenia: 2
- Pneumonia, pleural effusion, and acute respiratory failure: 2
- Abdominal pain and vomiting: 2
- Sepsis/septic shock: 2
- Intestinal obstruction and perforated appendicitis: 1
- Unspecified: 7
Two patients (1.1%) were hospitalized, with one due to severe anemia and the other due to a pathologic spinal fracture.
Although the number of outcomes (emergency room visits and hospitalizations) were limited, the authors were able to demonstrated that a worse ECOG performance status was significantly associated with an increased odds of emergency room visits (p=0.017) and hospital admissions (p=0.007).
Dr. Abdelrazek concluded that:
- Mild to moderate short-erm side effects secondary to Lu-PSMA treatment are not uncommon
- Most patients can be managed in an outpatient setting
- A worse ECOG performance status was associated with increased odds of emergency room visits and hospital admissions
- Larger studies are needed to further characterize the side effects/adverse event profiles of Lu-PSMA treatment in the real-world setting.
Presented by: Ahmad Abdelrazek, MBBCh, Research Fellow, Department of Radiology and Nuclear Medicine, Mayo Clinic, Rochester, MN
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting, Chicago, IL, Sat, June 24 – Tues, June 27, 2023.
References:- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021 Feb 27;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.


